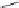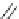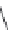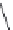Biomedical Engineering Reference
In-Depth Information
the enzymatic reaction; a recent survey suggested that more than 60% of marketed drugs that target
enzymes are either analogs of substrates or enzyme cofactors, or they undergo catalyzed structural
conversion within the active site of an enzyme. Substrate, cofactor, and product mimicry, however,
is not the only method for the design of high-afi nity, selective enzyme inhibitors. Advances in tran-
sition state theory during the past three decades have helped to establish an alternative approach for
mechanism-based design: intermediate-state-based design (sometimes also referred to as transition-
state-based design). In this latter approach, inhibitors that mimic the steric and electronic features
of high-energy reaction intermediate states are designed to capitalize on the specii c interaction of
active site residues with the reaction intermediate. In the next two sections, cases for substrate
structure-based design and intermediate-state-based design will be discussed to exemplify inhibitor
design strategies that have led to successfully marketed products or clinical candidates.
11.4.1 S
UBSTRATE
S
TRUCTURE
-B
ASED
D
ESIGN
11.4.1.1 Nucleoside and Nucleotide Inhibitors of HIV Reverse Transcriptase
HIV reverse transcriptase (RT) is one of two main targets for antiacquired immunodei ciency syn-
drome (AIDS) therapy (the second target being the HIV protease;
vide infra
). The RT enzyme cata-
lyzes the synthesis of double stranded proviral DNA from single stranded genomic HIV RNA. Drugs
targeting HIV RT can be divided into two categories: (i) nucleoside and nucleotide RT inhibitors
(NRTIs), which are competitive with respect to the natural deoxynucleotide triphosphates (dNTPs)
and serve as alternative substrates for catalysis (resulting in chain termination); (ii) nonnucleoside
RT inhibitors (NNRTIs), which are allosteric, noncompetitive inhibitors that bind at a site distal to
the RT active site. NRTIs were the i rst class of chemotherapeutic agents to be utilized in the clinic
to treat AIDS patients and offer excellent examples of inhibitor design based on substrate mimicry.
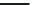
The i rst NRTI, Zidovudine (AZT) was approved by the FDA in 1987 (Figure 11.3).
This molecule is a thymidine analog with an azido group in place of the hydroxyl group at the 3
position of the ribose. Since the advent of AZT-based therapy, a number of NRTIs have joined the
′
O
NH
2
O
HN
N
NH
N
N
NH
N
OH
OH
OH
OH
N
O
O
N
N
N
O
O
N
NH
2
O
O
N
3
Abacavir
(Ziagen)
AZT
(Zidovudine)
ddC
(Zalcitabine)
ddl
(Didanosine)
NH
2
O
O
NH
2
N
N
N
NH
NH
N
OH
OH
OH
OH
O
N
N
N
N
O
N
NH
2
N
O
O
O
O
OH
OH
OH
OH
Deoxyadenosine
Deoxythymidine
Deoxycytidine
Deoxyguanosine
FIGURE 11.3
Representative FDA-approved nucleoside/nucleotide RT inhibitors (top panel) that closely
mimic the natural deoxynucleotides (bottom panel).