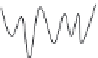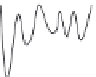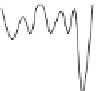Biomedical Engineering Reference
In-Depth Information
k
3
k
5
E + I
EI
E * I
k
4
k
6
(A)
EI
E*I
E
(B)
Microstate
Microstate
Microstate
E
EI
E*I
(C)
FIGURE 11.2
A two-step inhibitor-binding mechanism involving initial binding of the inhibitor to
the enzyme in one conformation and a subsequent isomerization of the enzyme to a new conformation.
(A) Reaction sequence illustrating the forward and reverse kinetic steps of binding and enzyme isomerization.
(B) Potential energy diagrams representing the three conformational states of the enzyme: E, EI, and E*I.
(C) Cartoon representation of the inhibitor binding and enzyme isomerization steps in this mechanism.
The state E*I then represents a state of high-afi nity interactions between the enzyme and
the inhibitor. As long as the inhibitor is bound to the enzyme, either in the form of EI or E*I, the
biological activity of the enzyme is blocked. Dissociation of the inhibitor from the enzyme can
occur for any reversible inhibition process; once the enzyme is free of inhibitor, catalytic activity
is restored. In the case of tight-binding inhibitors that induce enzyme isomerization, the overall
rate constant for inhibitor dissociation,
k
off
, must take into account reversal of the isomerization
step, reisomerization via
k
5
, and dissociation of the inhibitor from EI via
k
4
. Mathematically, the
value of
k
off
is given by
kk
46
k
=
(11.2)
(
)
off
kkk
++
3
5
6
For this two-step binding mechanism, it is almost always the case that the reverse isomerization
step, mediated by rate constant
k
6
, is by far the slowest step in overall inhibitor dissociation. Thus,
the lower the value of
k
6
, the longer the duration of potent inhibition by the drug. There are a large
and growing number of examples of highly efi cacious drugs that demonstrate tight-binding interac-
tions with their target enzyme through a two-step enzyme isomerization mechanism as described
here. In some cases, the slowness of the reverse isomerization step leads to prolonged duration of
inhibition that may translate into an extended duration of pharmacodynamic activity
in vivo
; this
concept is considered further in Section 11.6.
11.4 MECHANISM-BASED INHIBITOR DESIGN
Enzymes are designed by nature to catalyze a specii c chemical reaction. As described earlier, every
en zyme accesses a sequent ia l ser ies of inter med iate st ates a long t he react ion pat hway, t hus provid ing
unique opportunities for inhibitor interaction. Consequently, enzyme inhibitors can be effectively
designed based on an understanding of the mechanistic and structural details of the catalyzed reac-
tion pathway. The majority of known enzyme inhibitors are structurally related to natural ligands of