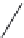Biomedical Engineering Reference
In-Depth Information
R
i
+1
R
i
+2
N
O
O
HN
HN
O
R
i
+3
R
i
O
NH
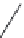
FIGURE 8.3
General structure of a β-turn.
a biologically active peptide has been identii ed, the next step is to determine the key amino acid
residues that are involved in the interaction with a receptor/acceptor via alanine scan, d-amino
acid scan, etc., and evaluation of the truncated sequences of the biologically active peptide as
described. Once the minimal peptide sequence and key amino acid residues have been determined,
the next step involves further modii cation of peptide conformation. There are two general strategies
to constrain the peptide conformations. The l exibility of a peptide chain can be restricted either by
global or local constraints.
(1)
Local conformational constraints
. Local conformational constraints can often provide
important insights into the structural basis of agonist, antagonist, and inverse-agonist
biological activity. The most informative local conformational constraints are those that
constrain the backbone
φ
,
ψ
, and
ω
torsional angles. Local constraints can be achieved by
-substituted amino acids, or modii cation of amino acid
side chains, and/or modii cation of the peptide backbone.
(2)
The modii cation of amino acid side chains
. If the conformational l exibility of the side
chain groups of key pharmacophore are restricted to varying degrees in a bioactive peptide,
important insights into their biologically active 3D topography can be obtained. Usually,
the side chain conformation can be controlled in several ways. One general approach is to
introduce an alkyl group at the
introduction of unnatural
α
- or
β
position of the aromatic
ring of an aromatic amino acid residue. These kinds of modii cations can constrain
β
-position or on the 3
′
and/or 5
′
χ
1
and
χ
2
angles; on the other hand, they generally do not perturb the backbone conformation
drastically, and still allow the peptides to have some degree of l exibility. In a similar
manner, substitution on the aromatic ring of an aromatic amino acid in the 3
′
and/or 5
′
positions will limit the conformational l exibility of a peptide to varying degrees depend-
ing on the nature of substitution. Furthermore, the introduction of alkyl groups, halogens,
or other functional groups can enhance the lipophilicity or other chemical properties and
thus help the peptide bind to receptors and/or cross membrane barriers. Incorporation of
these highly constrained amino acids into peptides and studies of such peptidomimetics
have provided a valuable approach to probe the stereochemical requirements of binding
pharmacophore for recognition of receptors, and sometimes such changes alone can lead to
completely different biological activities. Some examples from the Hruby research group
are given in Figure 8.4 as illustration of the many possibilities. Among natural amino
acids, proline is unique with a constrained cyclic system and substituted versions of this
amino acid can be used as a semirigid template in design of conformationally constrained
peptidomimetics.
(3)
The
modii cation of the peptide backbone
. Another strategy in the design of peptide drugs
is the peptide backbone modii cations, which generally refer to the isosteric or isoelec-
tronic exchange of NHCO units in the peptide chain or introduction of additional groups.
Some of the most frequent modii cations to the peptide backbone are listed in Figure 8.5.