Biomedical Engineering Reference
In-Depth Information
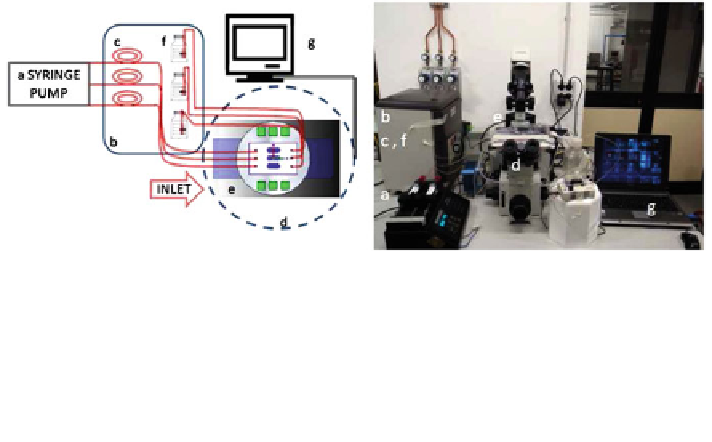
Fig. 7 Micro-bioreactor set-up developed to validate multiphysics computational models of
cartilage growth. Scheme (left) and photograph (right). a Syringes filled with complete cell
culture medium and mounted on an infusion/withdrawal programmable syringe pump, b cell
culture incubator (37C, 5% CO
2
), c silicone rubber oxygenator tubes, d inverted microscope,
e water-jacket heater used to maintain the micro-bioreactor chamber at 37C, f cell culture
medium reservoirs, and g laptop monitor showing a fluorescence image acquired directly on the
live micro-construct with a high resolution camera. A coloured version of this figure is available
on the online version of the topic
8 New Tools for Experimental Validation
In mechanobiology models of engineered cartilage, comparison between the
experimental findings and the computational results enables the local field vari-
ables to be correlated with specific cell responses, a crucial step towards model
validation. Generally this is a very complex procedure, as most variables to be
measured are not experimentally accessible in 3D and in real time. To attack this
problem, a first experimental set-up was developed and validated by our group in
collaboration with the University of Basel. A geometrically-defined custom made
scaffold was seeded with human chondrocytes and cultured inside a miniaturised
perfusion chamber, so-called mini-bioreactor, that permitted time lapse imaging of
the cellular construct in real time [
41
].
A step forward in this regard is a new optimized set up presented in Laganà and
Raimondi [
22
]. The new perfusion mini-bioreactor was designed and rapid-
prototyped taking advantage from microfluidic know-how, so it is easy to use, cost
effective, potentially disposable, and wholly mounted on a standard microscope
glass slide. The experimental set-up (Fig.
7
) is composed of a syringe pump,
a pipeline for cell feeding, a cell culture incubator and a fluorescence microscope
equipped with an imaging system. To house the custom-made polystyrene scaf-
folds used (3D Biotek), the perfusion chamber thickness was set at 300 lm,
i.e. over the traditional microfluidic scale (Fig.
8
).
The mini-bioreactor was successfully tested against leakage and used in cell
culture experiments. We have used live fluorescence viable staining, DAPI
(Sigma), in which the cell nuclei stain blue, and the Qtracker
Cell Labeling Kit
(Invitrogen), in which the cell cytoplasm stains orange, to follow tissue growth
Search WWH ::

Custom Search