Biology Reference
In-Depth Information
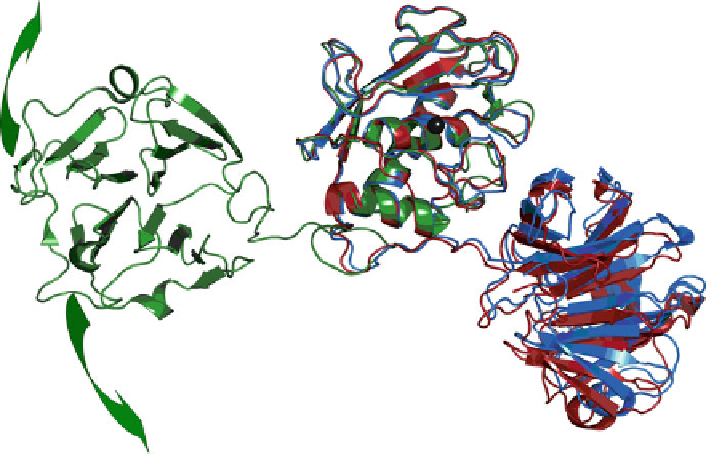
Fig. 6.6 The HPX domain freely and rapidly reorients relative to the catalytic domain in full-
length MMP-12 (
green
) (Bertini et al.
2008
) and to a lesser extent in full-length MMP-1 (
red
,
blue
)
(Bertini et al.
2009
,
2009b
). The HPX domain has the
-propeller fold at left and right. The
coordinates plot the catalytic domains (center) near the standard orientation using PDB accession
code 3BA0 for MMP-12, 2CLT for MMP-1 (
red
), and 1SU3 for the zymogen form of MMP-1
(
blue
). The zinc in the active site is plotted as a
black sphere
b
of which agree with the crystal structures of other full-length MMPs (Bertini et al.
2008
).
Full-length MMP-1 exhibited NMR relaxation evidence of similarly rapid
reorientation between the catalytic and HPX domains (Bertini et al.
2009
,
2009b
).
Moreover, the interface between two domains in the crystal structures of full-length
MMP-1 (Figs.
6.6
and
6.7
) was exposed to a probe molecule that broadens the NMR
peaks, suggesting that in solution the two domains were separated at least part of
their lifetimes (Bertini et al.
2009b
). Fits of SAXS data suggest that for about two-
thirds of their lifetimes MMP-1 molecules were similarly compact as the crystal
structure (red in Fig.
6.6
) (Bertini et al.
2009b
). The smaller proportion of extended
MMP-1 molecules, compared with MMP-12, might result from the linker being
two residues shorter in MMP-1 (Bertini et al.
2009b
). It could also be related to
the contacts between its catalytic and HPX domains that are absent in MMP-12
(Fig.
6.6
).
The linker between HPX and catalytic domains is longest in MMP-9. This length
promoted very loose tethering, judging from SAXS data and single-molecule
imaging. All dimensions of wild-type MMP-9 in solution were estimated to be
broad, such as the most populated 78
˚
distance between catalytic and C-terminal
lobes, whereas all dimensions were much smaller with the linker removed