Biology Reference
In-Depth Information
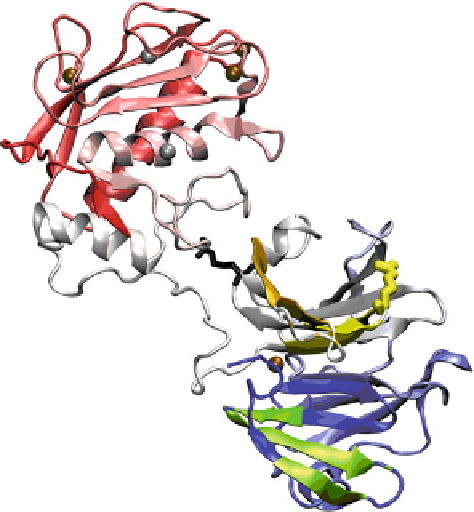
Fig. 6.7 Regions of MMP-1 (inactivated by E200A) with enhanced protection in HDXMS
experiments in the presence of a THP model of the MMP-1 cleavage in type I collagen (Lauer-
Fields et al.
2009
). The backbone ribbon color ranges from
red
at the N-terminus to
white
to
blue
at
the C-terminus. The protected peptide fragments from residue 287 to 295, 304 to 316, and 439 to
457, are colored
yellow
,
orange
-
yellow
, and
green
-
yellow
, respectively, upon the structure of the
active form of MMP-1 (Iyer et al.
2006
). The R291A substitution diminished MMP-1 activities
toward THPs and collagen I (Lauer-Fields et al.
2009
)
(Rosenblum et al.
2007
). These observations suggest that reorientation between
HPX and catalytic domains should be inherent to all MMPs in solution. This
reorientation should range from somewhat restricted in the collagenases with
shortest linkers (MMP-1, -8, and -13), to slightly restricted when the linker is a
little longer as in MMP-12, to largely independent in most MMPs, to maximally
independent in MMP-9.
6.4.3 Possible Implications of Loose Tethering for Proteolysis
of Fibrils
The longer and looser tethers of MMPs might serve simply to extend their reach
from the cell surface for pericellular proteolysis (Rosenblum et al.
2007
). 1D
diffusional locomotion was observed for MMP-1 traveling rapidly in one direction
along collagen fibrils (Saffarian et al.
2004
). In this context, loose tethering of