Biomedical Engineering Reference
In-Depth Information
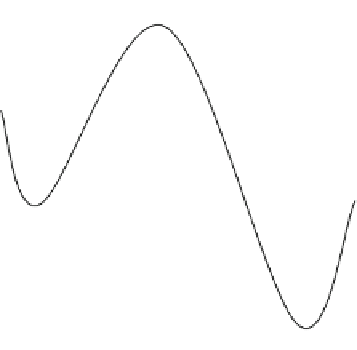
Fig. 5.25
Schematic illustration showing the free energy (F.E.) dependence on the reaction coor-
dinate (R.C.) when protein conformational transitions between different energy states occur. The
back-and-forth transitions between gA dimer (D) and monomers (M) have been demonstrated here.
These states have different energy values and are separated by a potential barrier.
G
I
,
II
(
Harm
)
and
energy terms are effective in the small deformation (bi-directional arrow in
the figure, within a very short range from the point of the energy minimum, thanks to harmonic
behavior) and beyond the small deformation region (
right arrow
), respectively, to ensure transitions
from D to M states and vice versa of gA. Only in the limit of extremely small bilayer deformation
(
d
0
G
I
,
II
(
A
.
Harm
)
2
may be sufficient.
−
l
∼
0) the inclusion of only the harmonic term
G
I
,
II
(
Harm
)
∼
(
d
0
−
l
)
When
d
0
l
increases beyond the immediate vicinity of the free energy minima, higher order
anharmonic energy terms dominate in the transition between D and M states. All such energy states
appear together in the screened Coulomb interaction model calculation but are missing in the elastic
bilayer model calculation of the bilayer deformation energy as explained in the text
−
determines the lipid curvature properties. Hence, the change in the lipid charge also
regulates the gA channel functions in lipid bilayers. The model calculation hints for
a stronger lipid charge effect on gA channel stability in the case of higher values of
d
0
−
l
(see Eq.
5.29
).
The experimental data for the Alm channel in a lipid bilayer also show consid-
erable agreement with the results of the theoretical model (see Figs.
5.17
and
5.18
).
To initiate the formation of an Alm channel under the experimental conditions men-
tioned in Sect.
5.4
, a minimum peptide concentration of
10
−
8
M is required [
12
].
However, once the Alm channel starts forming, we observe that the Alm channel
activity increases considerably with the increase of
∼
. These data are in good
agreement with the theoretical prediction of channel activity depending mainly on
[
M
Alm
]
G
I
,
II
in the initiation phase. Once the channels start forming, the bilayer's physical
parameters which determine
G
I
,
II
appear to be very critical in the regulation of the
channel formation mechanism and the channel formation rate sharply increases with
peptide concentration. We have also experimentally observed that the detailed Alm
channel activity shows considerable dependence on bilayer thickness and on lipid









Search WWH ::

Custom Search