Biology Reference
In-Depth Information
3.2.2
Chiral Assemblies of Monosubstituted Ferrocene-
Peptide Bioconjugates
The utilization of self-assembling properties of short peptides, which
possess chiral centers and hydrogen bonding sites, is considered
to be a convenient strategy to construct molecular assemblies.
Generally, an organized structure based on intramolecular interchain
hydrogen bondings is not formed in the case of ferrocenes bearing
only one peptide chain (Fig. 3.7), but a network of intermolecular
hydrogen bonds is expected to be formed in a solid state. The
ferrocene-peptide bioconjugate
bearing the one dipeptide
chain of the homochiral sequence (-L-Ala-L-Pro-OEt) exhibits
intermolecular hydrogen bondings between the NH of the Ala and
the CO adjacent to the ferrocene unit (another molecule), wherein
two independent molecules exist in an asymmetric unit and are
connected alternately to form an intermolecular hydrogen bonding
network, resulting in a left-handed helically ordered arrangement
with one turn of 17.86 Å pitch height and 7.50 Å separation (Fe-Fe)
between the closest ferrocene units (Fig. 3.8a) [8b]. The ferrocene
5
4
bearing only one dipeptide chain (-Gly-L-Pro-OEt) also forms a
hydrogen bonding network, in which each molecule is connected to
two neighboring molecules through intermolecular hydrogen bonds
between the NH of the Gly and the CO of the Gly (another molecule),
instead of intramolecular hydrogen bondings [19]. As observed in
the ferrocene
4
, the ferrocene
5
is packed in a left-handed helically
O
Me
O
N
OEt
N
OEt
H
H
O
O
O
O
Fe
Fe
Fc(-
L
-Ala-
L
-Pro-OEt)
1
4
Fc(-Gly-
L
-Pro-OEt)
1
5
O
Me
O
Me
N
N
N
N
N
N
H
H
O
O
O
O
Fe
Fe
Fc(-
L
-Ala-
D
-Pro-NH-2-Py)
1
6
Fc(-
D
-Ala-
L
-Pro-NH-2-Py)
1
7
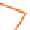
Figure 3.7
The ferrocene-peptide bioconjugates bearing one dipeptide
chain.








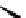



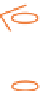



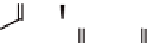






















Search WWH ::

Custom Search