Biology Reference
In-Depth Information
of 2D phosphonic acids alternate with nonpolar (NP)
-stacked
interdigitated pyrene moieties to form double interdigitated bilayers.
FTIR studies confirmed the presence of hydrogen bonds between the
phosphonic acids. This explains why the phosphonic acid headgroup
preferentially attaches to the hydroxylated SiO
π
surface. Hydrophobic
moieties form the layer exposed to air. Solvents with a slightly
longer alkyl chain resulted in imperfect structures with numerous
defect sites. A thin film of subhundred-nm grains developed on the
substrate after deposition from a polar, aprotic solvent (THF). Using
a more polar but still aprotic solvent (DMF) leads to clusters of PYPA
aggregates [3].
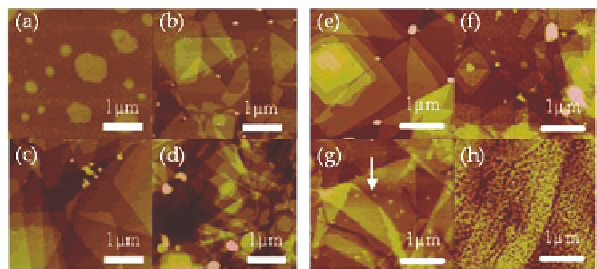
Concentration-dependent morphology changes were studied
using solutions of PYPA in ethanol. For the lowest concentration,
PYPA islands, consisting of a monolayer of the acid, could be
observed. Increasing the PYPA concentration resulted in increasing
layer thickness, due to the formation of stacked bilayers (Fig. 12.4)
[3].
2
Figure 12.4
Processing parameters influencing PYPA morphology.
AFM micrographs showing morphology in dependence of
(a)
−
−
(d) concentration in solution and (e)
(h) substrate. The
PYPA was spin-coated onto SiO
/Si substrates with ethanol
concentrations of (a) 0.1 mM, (b) 0.5 mM, (c) 1 mM, and (d)
2 mM. Structures resulting from the deposition of PYPA in
ethanol on hydrophilic (a) SiO
2
/Si, (b) glass substrates, (c)
inert Au/mica, and (d) hydrophobic HOPG. Reprinted with
permission from Ref. [3]. Copyright 2006 American Chemical
Society.
2
substrate surface interactions are expected to
determine whether the crystals formed in solutions can transfer and
Molecule
−


Search WWH ::

Custom Search