Biomedical Engineering Reference
In-Depth Information
changing osmolarities. However, if a toxicant is present in the sample at low concentra-
tions, a high dilution may make the substance no longer detectable. Concentration steps
are time consuming and may interfere with the efficacy of the toxicant. Therefore, the most
direct and rapid method is to mix samples with preconcentrated medium. Doubly con-
centrated medium has already been tested for long-term stability and utility, allowing a
50/50 mixture of medium and test sample. This approach was used to test a series of sam-
ples during a function-based assay workshop entitled Eilatox-Oregon, where the NNBS
successfully detected a range of environmental samples [7]. A mixture of three parts of
aqueous test sample with one part of concentrated medium is also possible, although
long-term medium storage has not been explored. With this approach the test sample need
only be filtered if microorganisms are to be excluded although short-term tests of acute
toxicity can be performed in the presence of microorganism. Clearly, if the microorganisms
are the target of the scrutiny, they will grow very well in culture medium and can be
explored optically and with a variety of staining methods.
One of the major misconceptions concerning the use of mammalian cells as detection
elements is the presumed lack of tolerance to chlorine, which is commonly used in water
purification. For water purification, chlorine levels reach 2-4 ppm and must be considered
a potential interferent in the analysis of water toxicity. The University of North Texas
(UNT) group has thoroughly examined this issue with respect to the NNBS, finding vir-
tually no alteration in the pharmacological responsiveness of the networks in the presence
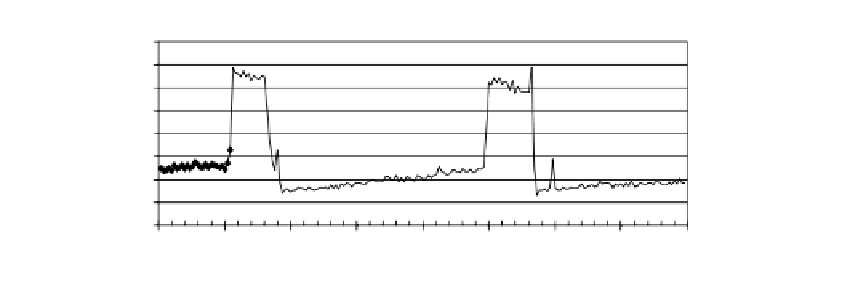
of high levels of chlorine. Figure 6.19 shows the effect of a synaptic disinhibitor, TMPP, in
the absence and presence of 10 ppm chlorine, a concentration well above that which is
used in water purification. In fact, networks grown and maintained in DMEM (frontal cor-
tex) and MEM (spinal cord) with 5% serum withstand acute additions of up to 20 ppm
chlorine without suffering damage. This protection was linked to serum concentrations
and specifically to serum albumin [36].
6.4.3
Portable Sensor Units
Efforts to develop a portable cell-based biosensor have been ongoing for nearly a decade [37,
38, 8]. The major interest in this area has been the recognition that current sensor paradigms
for environmental threats are incapable of detecting unanticipated threats, raising the need
for a broad-spectrum detection capability [6]. The initial design philosophy centered on
40
M
TMPP
µ
40
µ
M
TMPP
800
45
+10 ppm
chlorine
40
700
2X Wash
2X Wash
35
600
30
500
25
400
Spike rate
Burst rate
20
300
15
200
10
100
5
0
0
0
25
50
75
100
125
150
175
200
Time (min)
ED27 FC 26div n=7 channels
FIGURE 6.19
Stable baseline spike and burst rates increase over twofold upon addition of 40 µM TMPP. Two full medium
changes with DMEM-5 restore baseline activity (wash). Treatment with 10 ppm chlorine (trichlor) at 92 min
(arrow) does not produce a marked effect, and a subsequent TMPP application under 10-ppm chlorine elicits the
stereotypical TMPP response in this culture.