Geology Reference
In-Depth Information
SO
2
Mg
100
50000
80
10000
5000
60
1000
40
500
20
100
0
(a)
0
30
60
90
120
Mg
100
50000
80
10000
5000
60
1000
40
500
20
100
20 40 60 80 100 120
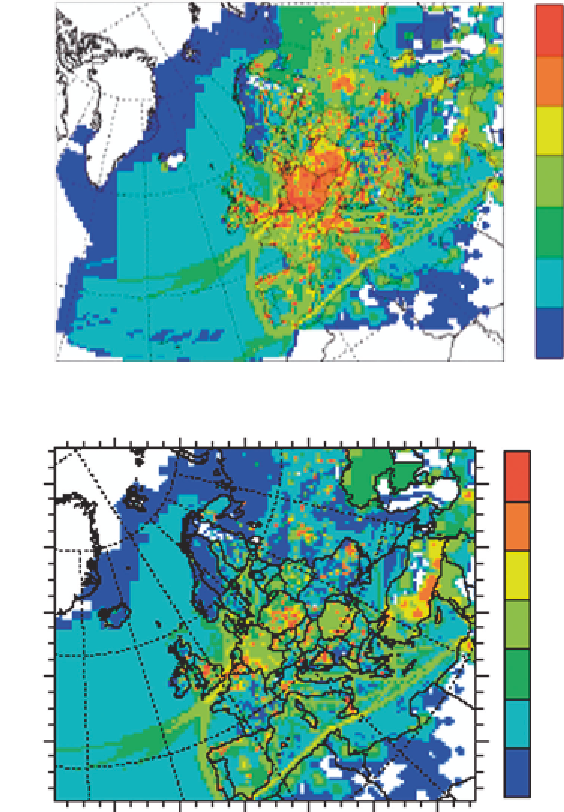
Figure 19 Spatial distributions of sulfur dioxide emissions in (a) 1980 and (b) 2000.
34
The units are ton year
1
grid sq
1
(b)
aerosol particles. The bulk of the H
2
SO
4
is lost via wet deposition
mechanisms in cloud droplets and precipitation. There is another po-
tential gas-phase loss route for SO
2
that can lead to the formation of
sulfuric acid in the presence of H
2
O, i.e. the reaction of SO
2
with Criegee
intermediates (see Section 2.7). The aqueous-phase oxidation of SO
2
is