Geology Reference
In-Depth Information
more complex, depending on a number of factors such as the nature of
the aqueous phase (e.g. clouds and fogs), the availability of oxidants
(e.g. O
3
and H
2
O
2
) and the availability of light. An overview of the
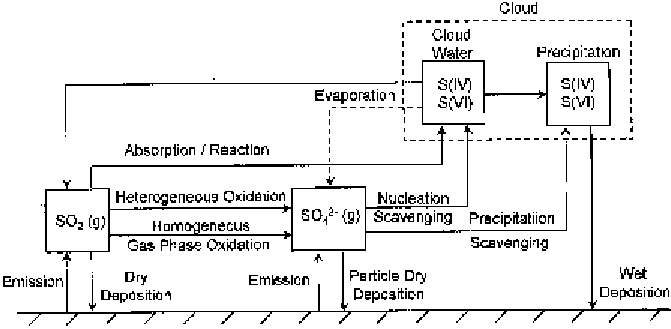
mechanism is given in Figure 20. The key steps include the transport of
the gas to the surface of a droplet, transfer across the gas-liquid
interface, the formation of aqueous-phase equilibria, the transport
from the surface into the bulk aqueous phase and subsequent reac-
tion. In brief, the SO
2
gas is dissolved in the liquid phase, establishing
a set of equilibria for a series of S(IV) species, i.e. SO
2
H
2
O, HSO
3
and SO
3
2
.
SO
2
(g)
þ
H
2
O
"
SO
2
d
H
2
O(aq)
(2.69)
HSO
3
þ
H
1
SO
2
d
H
2
O(aq)
"
(2.70)
HSO
3
SO
2
3
þ
H
1
"
(2.71)
The solubility of SO
2
is related to the pH of the aqueous phase,
decreasing at lower values of pH. The oxidation of sulfur (IV) to sulfur
(VI) is a complex process dependent on many physical and chemical
factors. The main oxidants seem to be O
2
(catalysed/uncatalysed), O
3
,
H
2
O
2
, the oxides of nitrogen and free-radical reactions in clouds and
fogs. For example, H
2
O
2
is highly soluble in solution so even at rela-
tively low gas-phase concentrations (typically ca. 1 ppbv) there is a
Figure 20 Summary of emission, oxidation and deposition of S(IV) and S(VI) (after
ref. 63)