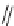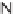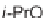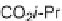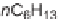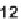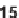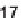Biomedical Engineering Reference
In-Depth Information
In the tandem Diels-Alder/acylation sequence approach to building small focused
libraries (Scheme 3.5), Aube et al. began with the hypothesis that the Diels-Alder
cycloaddition of highly reactive dienophiles with amine-containing dienes would
give only the endo product, with the amine side chain in a cis orientation with respect
to the reactive carbonyl group, thus promoting the acylation step. Following the
optimization of reaction conditions, a one-step synthesis of an isoquinolone (
10
)
containing a carboxylic acid for further diversification was achieved. In contrast with
the authors' original hypothesis, they did not find any evidence that the reaction pro-
ceeds by the initial Diels-Alder cycloaddition reaction followed by the intramolecular
acylation.
Recent work by Cui et al. demonstrated the power of combining several reactions
to achieve both skeletal diversity and structural complexity (Scheme 3.6) [16]. The
Diels-Alder cycloaddition reaction was used along with 1,6-enyne cycloisomeriza-
tion, Os-catalyzed dihydroxylation, and Sn-catalyzed carbamoylation in a unique
approach that generated 191 skeletally and structurally diverse compounds with high
diastereoselectivity and employing only 16 building blocks. Principal moment of
inertia (PMI) computational analysis showed that the library covers a broad chem-
ical space, suggesting shape diversity [17]. The 1,3-dienes (
11-14
) were synthe-
sized through transition metal-catalyzed cycloisomerizations of 1,6-enynes. Four
dienes were reacted with five selected dienophiles (
15-19
) with high efficiency and
SCHEME 3.6
Diels-Alder cycloaddition reactions of dienes generated through transition
metal-catalyzed cycloisomerizations of 1,6-enynes.