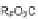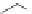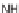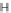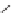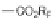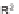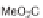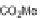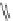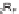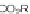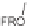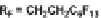Biomedical Engineering Reference
In-Depth Information
SCHEME 1.1
DOS of a library of small molecules from a simple diazoacetate starting
material
1
. Step 1 refers to the first step of the DOS, Step 2 refers to the second step of the
DOS. Reagents and conditions: (a) C
6
H
6
,Rh
2
(OCOCF
3
)
4
; (b) R
1
CCH, Rh
2
(OAc)
4
,CH
2
Cl
2
;
(c) thiophene, Rh
2
(OAc)
4
; (d) furan, Rh
2
(OAc)
4
then I
2
;(e)LDA
78
◦
C then R
2
COR
3
,
THF then Rh
2
(OAc)
4
,CH
2
Cl
2
; (f) DMAD; (g) PhCHO, PhNH
2
then DMAD, Rh
2
(OAc)
4
or
PhMe [Cu(OTf)]
2
,CH
2
Cl
2
; (h) methyl acrylate; (i) R
4
NH
2
, NaOH, H
2
O, 180
◦
C then MeOH,
H
2
SO
4
,60
◦
C; (j) dienophile, toluene, reflux; (k) DMAD, toluene, 100
◦
C; (l) cyclopentadiene,
CH
2
Cl
2
,0
◦
C to rt; (m) Grubbs's second-generation catalyst, toluene, ethylene, reflux; (n)
phenol derivative, conc. H
2
SO
4
; (o) guanidine, EtOH, reflux; (p) guanidine, R
6
CHO, DMF,
75
◦
C; (q) NH
2
OH, THF, reflux; (r)
m
CPBA, CH
2
Cl
2
, rt; (s) substituted 3-formyl chromone,
EtOH, reflux; (t) substituted 3-formyl chromone, EtOH, reflux.
−
The first library, synthesized in 2006 by Wyatt et al., used a fluorous-tagged dia-
zoacetate species (
1
) as a two-carbon starting unit (Scheme 1.1) [46]. This compound
can be considered to be pluripotent, as under a range of conditions it is able to act
as both a nucleophilic and an electrophilic species. In total, a library of 223 small
molecules was synthesized, based around 30 distinct molecular skeletons. This syn-
thesis was achieved in two to four synthetic steps from the diazoacetate species,
clearly exemplifying the powerful nature of this type of approach to molecular diver-
sity generation.
An initial three-way branching strategy was employed, involving three-membered
ring formation by addition of rhodium carbenoids to alkene species;
-deprotonation