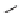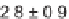Biomedical Engineering Reference
In-Depth Information
FIGURE 9.8
Selected examples of diverse cyclic compounds with specific biological func-
tions.
The cyclization reactions of
N-
acyl iminium ions have yielded a wide range of
distinct small-molecule scaffolds (Scheme 9.21) [45]. Initially, peptide synthesis
was used to connect a range of functionalized amino acid building blocks. Each of
the resulting peptides,
181
, was designed to contain a masked aldehyde, a suitably
positioned secondary amide, and a pendant nucleophile. Treatment of the peptides
with acid triggered the release of an aldehyde, the formation of an
N-
acyl iminium
ion, and cyclization to yield a final scaffold (e.g.
182
to
187
).
Metathesis cascades involving oligomeric substrates have underpinned the synthe-
sis of skeletally diverse small-molecule libraries [46-48]. Within the Nelson group,
for example, we prepared a wide range of oligomeric metathesis substrates by
the iterative attachment of unsaturated building blocks to a fluorous-tagged linker
(Scheme 9.22) [47]. Crucially, alternative attachment reactions were used such that