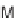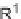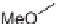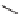Biomedical Engineering Reference
In-Depth Information
SCHEME 9.20
Parallel solution-phase synthesis of pyran-containing macrocycles: (a) Si-
DCC, HOBt, DIPEA, CH
2
Cl
2
; Si-CO
3
;b)TFA,CH
2
Cl
2
; Si-CO
3
,3:1CH
2
Cl
2
-MeOH; (c)
Cs
2
CO
3
, DMF, 110
◦
C; Si-CO
3
,CH
2
Cl
2
; 16 to 29%, yields over four steps.
approach also enabled the synthesis of pyran-containing macrocycles (Scheme 9.20)
[42b]. By exploiting pyrans
169
and
170
as building blocks, a wide range of macro-
cycles (e.g.,
175
and
176
) was prepared using nucleophilic aromatic substitutions
in the macrocyclization step. The synthetic approach has enabled the discovery of
a range of cyclic molecules with diverse biological functions (Figure 9.8) [42a,43].
Other researchers have also exploited oligomer-based approaches to prepare diverse
macrocyclic scaffolds [44].