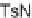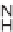Biomedical Engineering Reference
In-Depth Information
FIGURE 9.7
Chemical structure of 146, a suppressor of glycolytic ATP production.
branching approach utilized the common enyne
122
, which could be cycloisomerized
to yield alternative 1,3-dienes (
123
to
125
). These were reacted with dienophiles to
yield a diverse array of mono- and polycyclic compounds, which were then dihydrox-
ylated and functionalized (not shown) to give a 191-member library. From this library,
the compound
146
was identified as a suppressor of glycolytic ATP production in
CHO-K1 cells (Figure 9.7).
Robbins et al. have prepared a wide range of natural product-like scaffolds (
148
to
159
) using an outstanding branching pathway (Scheme 9.18) [34,35]. The approach
exploited the remarkable chemistry of the symmetrical ketone
147
, which has two
appended unsaturated esters. The approach takes advantage of a wide range of reac-
tions, including conjugate additions (e.g., of amines, enolates, and ketyl radicals)
and dipole formation and cycloaddition. Other branching pathways leading to natu-
ral product-like molecules have exploited Diels-Alder reactions [36], boronic ester
chemistry [37], diazoester chemistry [38], the diverse chemistry of
-unsaturated
carbonyl compounds [39], cyclizations of
N
-allyl and
N
-propagyl propargylic amine
derivatives [40], and branching cascade chemistry [41].
,
9.5 OLIGOMER-BASED APPROACHES TO NATURAL
PRODUCT-LIKE LIBRARIES
An extremely powerful strategy for preparing small-molecule libraries with wide
scaffold diversity exploits simple building blocks in combination. The approach
requires the building blocks to be prepared (“built”) and then connected (“coupled”).
Finally, pairs of functional groups are reacted (“paired”) intramolecularly to yield
new ring systems in the final scaffolds. This
build/couple/pair strategy
which has
been reviewed by Nielsen and Schreiber [9e], is extremely broad in scope. Indeed,
some folding pathways and branching pathways can be considered to exemplify the
build/couple/pair strategy (e.g., the synthetic approach described in Schemes 9.12
and 9.13).
Marcaurelle's group has used an oligomer-based approach has been exploited to
prepare skeletally diverse macrocycles (Schemes 9.19 and 9.20) [42]. The approach
involved the assembly of cyclization precursors from combinations of simple build-
ing blocks. For example, stereoisomeric amines
162
were prepared from the amines