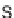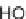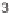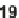Biomedical Engineering Reference
In-Depth Information
(a)
(b)
FIGURE 9.5
Discovery of protein phosphatase inhibitors inspired by scaffolds based on
indole alkaloids: (a) chemical structures of yohombine, ajmalicine, and reserpine, which inhibit
the dual specificity phosphatase Cdc25A (IC
50
: 22, 32, and 64
M, respectively); (b) chemical
structures of inhibitors of the tyrosine phosphatase MptpB (IC
50
: 18, 1.1
M; 19, 0.36
M;
20, 0.43
M).
the value of hierarchical scaffold classification, which can facilitate effective navi-
gation (between related molecular scaffolds) within biologically relevant chemical
space.
9.3 FOLDING PATHWAYS IN THE SYNTHESIS OF NATURAL
PRODUCT-LIKE LIBRARIES
The folding pathway [22] approach exploits common reaction conditions to transform
a range of substrates into products with alternative molecular skeletons (Scheme 9.4).
The substrates are encoded to “fold” into the alternative scaffolds through strategically
placed appending groups, often referred to [22] as
-elements. This strategy allows
variation of the molecular scaffold using a single set of reaction conditions.