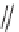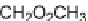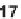Biomedical Engineering Reference
In-Depth Information
FIGURE 9.4
Chemical structure of macroline.
releasing the final compounds from the solid support. The substitutional diversity
of the library was increased by exploiting functionalization reactions before release
from the solid support.
Noren-Muller et al. exploited natural product-inspired chemical libraries in the
discovery of novel inhibitors of protein phosphatases (see Figure 9.5) [13a]. Medium-
sized compound libraries were prepared on the basis of simplified scaffolds related to
those found in specific natural products. As an illustrative example, libraries inspired
by yohombine, ajmalicine, and reserpine were prepared in which the complex
polycyclic framework had been simplified. From these libraries, inhibitors of a range
of protein phosphatases were discovered, including
18
to
20
, in which the core is bi-,
tri-, or tetracyclic. For two specific phosphatases, the first inhibitors were thereby
discovered (e.g.,
18
to
20
, which inhibit MptpB). This overall approach demonstrates
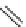
SCHEME 9.3
Synthesis of macroline-inspired compounds: (a) ArCHO, 1 : 1 HC(OMe)
3
-
CH
2
Cl
2
(1 : 1); (b) NaCNBH
3
, 20% AcOH in THF; (c) TFA in CH
2
Cl
2
×
2; (d) methyl-4,4-
dimethoxybutanoate, 15% TFA in CH
2
Cl
2
; (e) 15% Et
3
NinCH
2
Cl
2
×
2; (f) 1 M NaOMe, 1 :
1 MeOH-1,4-dioxane; (g) NaOMe, 10% MeOH in PhMe, reflux.