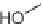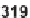Biomedical Engineering Reference
In-Depth Information
conditions. In both cases the final products were released from the support by treat-
ment with DDQ. Alternatively, oxidation with Dess-Martin reagent afforded pyrones
309
, which were treated with binucleophiles to afford thiazinones
310
or benzoth-
iazinones
311
. These products were cleaved from the resin under acidic conditions.
Precursor
305
was also esterified or etherified. While ester
312
was released from
the resin with DDQ, ether
313
was either cleaved under the same conditions or was
further derivatized to deoxysugar derivative
314
. It was either cleaved from the resin
as a final compound or was reduced prior to the cleavage to yield tetrahydro-2
H
-
pyran-2,5-diol
315
. Finally, intermediate
313
was also treated with 4,7-dimethoxy-3-
oxodihydroisobenzofuran-1-carbonitrile to afford naphthoquinone
316
under acidic
cleavage from the solid support.
Alternatively, scaffold hopping was utilized for the preparation of two diverse scaf-
folds by Diels-Alder reaction. The key precursor
319
was achieved by extraction from
several plants, such as
Piper nigrum
,
Eugenia caryophyllata
, and
Pimenta dioica
,in
organic solvent in the presence of resin-bound nitroso compound
317
(Scheme 7.43)
[80]. The resin selectively sequestered only one of the substances extracted, the
-
caryophyllene
318
. Resin-bound ene product
319
was converted to two structurally
SCHEME 7.43
Synthesis of diverse scaffolds from natural product extracts. Reagents and
conditions: (i) DCM, 1 h; (ii) 0.1 M TBAF, THF, rt, 30 min (iii) 50% TFA/DCM, rt, 1 h.