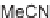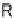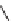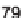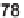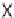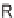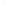Biomedical Engineering Reference
In-Depth Information
TABLE 4.10 Benzannulated Heterocycles
Entry
R
1
R
2
X
Y
Yield (%)
1
O
S
H
H
93
2
O
O
H
H
80
3
S
S
H
H
74
4
S
NTs
H
H
68
5
NTs
NTs
H
H
79
6
O
NTs
H
H
84
7
O
S
Ph
H
86
8
O
O
Bn
H
89
9
O
O
H
Me
89
10
a
O
NTs
H
CH
2
CO
2
Et
84
b
a
NaOAc (50 mol%) was added.
b
dr
1.2 : 1, based on the
1
H NMR spectrum of the crude reaction mixture.
=
acid-derived amino alcohols, thiols, malonates, and malononitriles as Michael donors
and electron-deficient acetylenes as Michael acceptors provided efficient access to
enantiomerically pure oxazolidines, thiazolidines, pyrrolidines, and octahydroin-
doles in excellent yields with high diastereoselectivities [59,61]. In the mechanism
proposed, the bisphosphine [e.g., diphenylphosphinopropane (DPPP)] stabilizes the
intermediate phosphonium ions and improves the reaction efficiency (Scheme 4.24).
Switching to aromatic dinucleophiles, we also prepared seven distinc-
tive heterocyclic compounds—indolines, dihydropyrrolopyridines, benzimidazo-
lines, dihydrobenzo-3,1-oxazines, benzomorpholines,
tetrahydroquinolines, and
SCHEME 4.24
Phosphine-catalyzed double-Michael reaction.