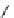Biomedical Engineering Reference
In-Depth Information
X
E
cat. DPPP
XH
E
+
NHTs
MeCN
reflux or rt
N
Ts
75
77
76
SCHEME 4.25
Synthesis of 11 different heterocyclic scaffolds.
tetrahydroisoquinolines—through the mixed double-Michael strategy. In some cases,
the use of acetic acid and sodium acetate as additives expedited the proton transfer
steps and, consequently, the overall reaction (Scheme 4.25 and Table 4.11) [60]. In the
realm of phosphine organocatalysis, the mixed double-Michael reaction of acetylenes
and dinucleophiles appears to be the most versatile annulation process for generating
heterocyclic compounds.
4.3 SKELETAL DIVERSITY BASED ON A PHOSPHINE
CATALYSIS/COMBINATORIAL SCAFFOLDING STRATEGY
The algorithm of DOS (i.e., in split-and-pool format) manifests two contradicting
principles in operation: maximizing structural diversity and maintaining common
reactivity. For the sake of diversity, one must generate structures that are as different
as possible. This diversity requirement alone is not particluarly challenging and
quite manageable [26-36]. Examples have appeared in the literature since the 1990s,
such as Armstrong's proposal that squaric acid could be used as a fluid core system
to generate a multiple-core structure library. The result could be eight drastically
different core structures (Scheme 4.26) [13]; these eight compounds, however, cannot
be pooled and subjected to the next split step, because they do not share any common
chemical functionality to exploit. In this case, maintaining common reactivity has
been sacrificed for the sake of diversity.
This quandary demonstrates the challenge associated with more advanced forms
of DOS: combinatorial scaffold generation. To satisfy this high standard of com-
binatorial scaffolding, we have developed new phosphine catalyses of allenoates
to produce a diverse array of compounds, including dihydropyrroles, tetrahy-
dropyridines, cyclohexenes, bicyclic succinimides, dioxanylidenes, tetrahydropyra-
zolopyrazolones tetrahydropyrazolopyridazinones, tetrahydropyrazolodiazepinones,
and tetrahydropyrazolodiazocinones [46-48,50,51,53]. This set of compounds is as
diverse as Armstrong's, yet, by design, only one unit of unsaturation in the allenoates
is consumed, leaving the versatile
-unsaturated ester moiety as a common chem-
ical functionality to be exploited in the next split step after pooling all 11 scaffolds.
Exploration of this common chemical functionality (
,
-unsaturated ester) in the
second scaffold-generating step (Michael addition or Tebbe/Diels-Alder reaction) is
one of the key features of our library design: combinatorial scaffolding. In combina-
torial scaffolding, diverse products generated from a set of phosphine organocatalysis
reactions in one split step are programmed to contain a common chemical functional
group (e.g.,
,
-unsaturated ester) so that they can be pooled and subjected to the
next scaffold-generating split step.
,