Biomedical Engineering Reference
In-Depth Information
O-H
1550
(a)
1425
1460
CO
3
CO
3
PO
4
PO
4
(b)
867
1530
1470
(c)
879
869
1465
1420
3500
2500
1500 1200
cm
-1
1000
800
600
400
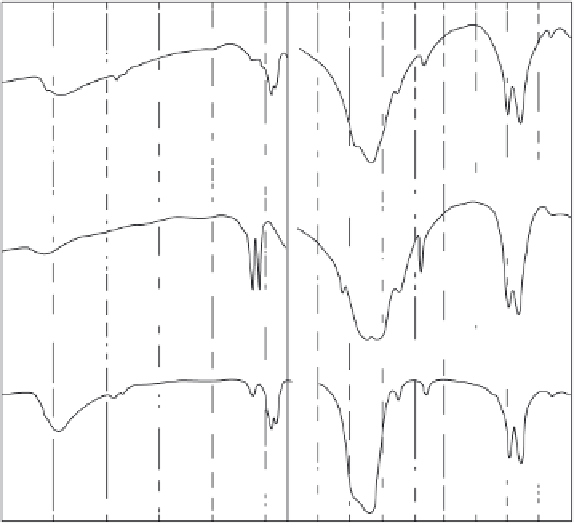
Figure 2.4.
IR spectra of synthetic carbonate apatite, Type B (C) and Type A (B) compared to
that of biologic apatite, enamel apatite (C). The spectra of Type B carbonate apatite, CO
3
-for-
PO
4
coupled with Na-for-Ca (A) is more similar to that of biologic apatite (C) [21,57,59,64,83].
larger crystals (similar to tooth enamel apatite) are obtained by precipitation or
hydrolysis method at 80 to 95 °C [57,59,64]. Larger size crystals are obtained by
hydrothermal reactions (Figure 2.5) [41]. Single crystals of carbonate apatite with
one or both type of CO
3
substitution are obtained from high pressure solution
growth methods, typically, hydrothermal and fl ux methods (Table 2.4 ).
Solubility of the apatite increases as the amount of carbonate in the apatite
increases regardless of the type of substitution: Type A [40] or Type B [73]. In-
corporation of one
CO
2−
per unit cell in hydroxyapatite by the CO
3
- for - OH
substitution increased the solubility product by 10
15.9
[40] .
X-ray structure analysis using fl ux-grown carbonate apatite single crystals
revealed that planar CO
3
in CO
3
- for - PO
4
substitution are located close to the
sloping oxygen triangle consisting of the O(1), O(2) and O(3) of the PO
4
group in
hydroxyapatite [25,26] confi rming earlier inferences from polarized IR study [22].
The sloping angle that is defi ned by the angle between the normal to the CO
3
plane and the
c
-axis varied depending on the Na-substitution of adjacent Ca site.
Planar CO
3
groups in CO
3
-for-OH substitution are located at the height nearly
the same as that of Ca (I) (
z
= 0, 1/2) or hydroxyapatite with the CO
3
plane being
parallel [115] or nearly parallel (canting less than 12 °) to the
c
- axis [Ito - 16,17].
Search WWH ::

Custom Search