Biomedical Engineering Reference
In-Depth Information
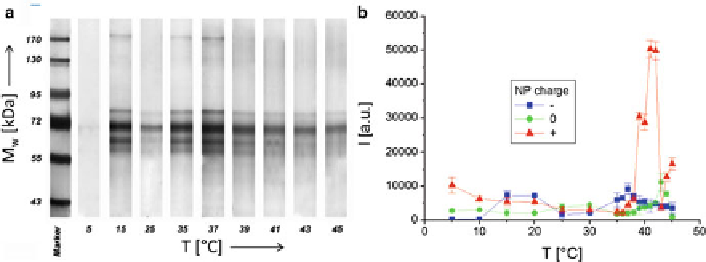
Fig. 2.5
(
a
) SDS-PAGE gel of proteins adsorbed onto the surfaces of negatively charged Fe
3
O
4
NPs after 1 h incubation in FBS at different temperatures
T
. The molecular weights
M
w
of the
proteins in the marker lane on the
left
are reported for reference. (
b
) Quantification of the amount
of adsorbed proteins on negatively charged (
), neutral (0), and positively charged (+) NPs as
derived from the total band intensities of proteins on the SDS-PAGE (one-dimensional sodium
dodecyl sulfate polyacrylamide gel electrophoresis) gels (adapted with permission from [
32
])
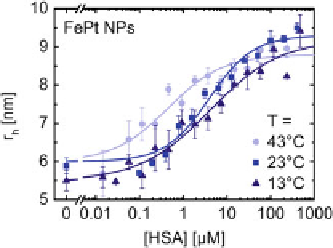
Fig. 2.6
Dependence of the hydrodynamic radius of negatively charged FePt NPs on the concen-
tration of HSA in the solution due to protein adsorption at 13, 23, and 43
C (adapted with
permission from [
32
])
The fluorescently labelled, negatively charged polymer-coated FePt were also
employed for evaluation of the attachment of Human serum albumin (HSA) to their
surfaces using fluorescence correlation spectroscopy (FCS) [
33
]. The HSA were
incubated with FePt nanoparticles for 10 min at different adjusted temperatures (
T
);
then, the fluorescence were measured with the FCS setup for 4 min at the same
temperature
T
. Hydrodynamic radii
r
h
as determined with FCS were plotted versus
the HSA concentration in solution,
c
(HSA) (see Fig.
2.6
).
N
is the number of
adsorbed HSA molecules per NP, and
N
max
is the maximum number of adsorbed
molecules. At saturation,
the hydrodynamic radius of one NP is calculated
according to
p
3
r
h
ð
N
max
Þ¼
r
h
ð
0
Þ
1
þ
c
N
max
(2.1)