Biomedical Engineering Reference
In-Depth Information
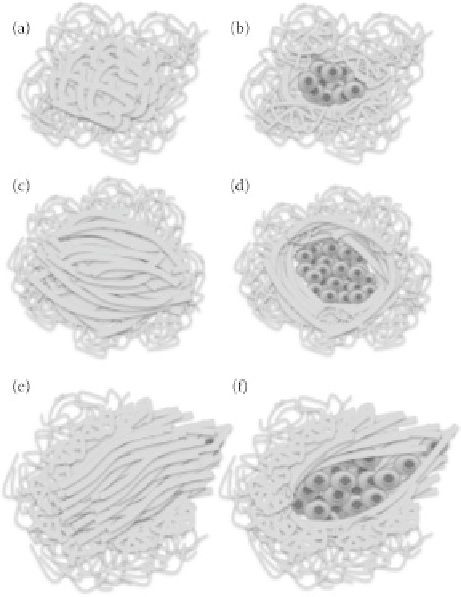
FIgurE 16.2
Graphical representations of tumor associated collagen I structures as described in Table 16.1.
(a-b) Category 1, (c-d) Category 2, (e-f) Category 3.
a research target. Likewise, it is only in the last few years that collagen has emerged as a possible clinical
target. The challenges of considering collagen as a clinical target are largely due to the properties of col-
lagen: it is intricate in design, dynamic, abundant, needed for normal function and interwoven with a net-
work of other proteins, cellular components, and processes. Even more challenging is that its properties
are regulated by other constituents in the ECM and by the physical and chemical conditions under which
it is assembled. Thus, collagen polymerized
in vitro
is composed of largely uniform, thin, short fibers and
differs from that deposited
in vivo,
which is characterized by a variety of fiber thicknesses and lengths
.
To
truly understand collagen in its
in vivo
functions, whether its beneficial role as a structural protein or a
hijacked player in breast cancer invasion and progression, it is necessary to have a method that can moni-
tor all aspects of collagen in the most physiologically relevant and realistic microenvironment possible.
Accomplishing this goal of monitoring and studying the role of collagen in human breast cancer
necessitates a very specific type of imaging modality. An effective imaging tool for studying collagen-
affiliated processes in breast cancer ideally must: (1) have sufficient resolution and specificity for detect-
ing collagen, normal cells, and cancer cells; (2) be able to detect intrinsic and extrinsic labels for cellular
components and cells; (3) have the ability to track over time; and (4) be able to noninvasively image deep
into intact tissue.
The quest for early detection and treatment to improve survival rates of cancer is the driving force
behind the development of accurate optical biomarkers. Changes in cellular behavior, phenotype,
physicochemical properties or alterations to the ECM are all detectable by optical imaging modalities.
When quantified in research settings, optical biomarkers have shown potential for earlier detection of
carcinoma lesions. Optical biomarkers are innate to the tissue, providing a method to observe natural
cellular activities and to correlate underlying biological changes to the marker.