Biomedical Engineering Reference
In-Depth Information
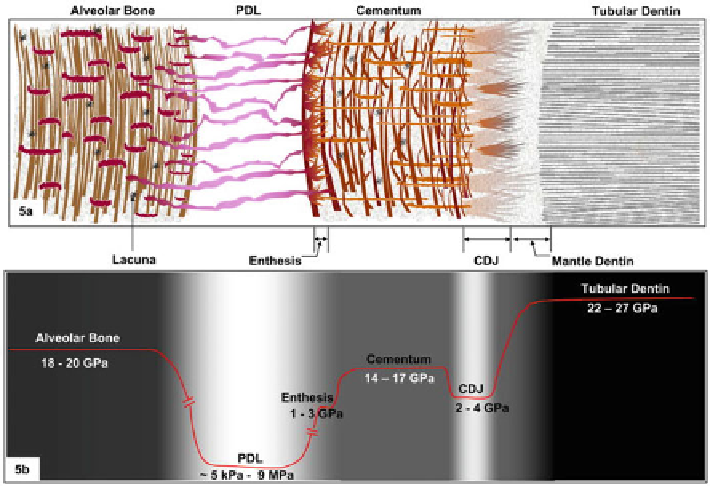
Fig. 1.7
Schematics of the tissues and interfaces responsible for tooth attachment. (
a
) Structure
and (
b
) variation of the modulus of elasticity, reflecting the presence of graded regions between
bone, PDL, cementum, and root dentin (adapted, with permission, from [
9
])
Notably, the surface structure, in particular the surface roughness, and the surface
chemistry of oral implants influence their anchoring in jaw bones. In one study, the
metal-to-bone interface between an implant surface and a jawwas modified by sand-
blasting the metal component with different particles [
10
]. The surface modification
of the titanium implant was produced by blasting it with particles of Al
2
O
3
or of
bioceramics; these treatments resulted in improvements in attachment. This
illustrates that an artificially developed roughness on the implant surface may benefit
attachments for tooth implants. Nevertheless, caution should be exercised using this
approach, since local damage may be introduced in the material during the blasting
process. Additionally, the mechanisms by which this improvement is attained are
not understood. The presence of local roughness may serve as a source of micro-
scopic stress concentrations; a macroscopic crack may originate from one of these
local areas. Alternatively, the roughness might serve as a cue for living cells within
the jaw to produce material more conducive to an effective metal-to-bone interface.
1.2.2.2 Metal-to-Bone Orthopedic Interfaces
Metal-to-bone interfaces in orthopedic total joint implants have been extensively
investigated. A mismatch in the stiffness along the interface can cause both pain
and eventual loosening or failure, requiring replacement of the artificial joint. A
second issue that can lead to negative outcomes is the biocompatibility of the metal.
Search WWH ::

Custom Search