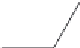Biomedical Engineering Reference
In-Depth Information
Figure 2.1
PEEK production facility, Thornton-Cleveleys, United Kingdom. Source: Victrex.
polymers from being synthesized in common organic
solvents. Early attempts to synthesize PEEK in
methylene chloride or nitrobenzene produced only
low-molecular-weight variants.
Work by DuPont using a combination of anhy-
drous hydrogen fluoride/boron trifluoride succeeded
in protonating the carbonyl groups and meant that
high-molecular-weight
acid to form PEEK. This route has only remained of
academic interest due to the extremely high cost and
corrosive nature of the solvent used (
Scheme 2.2
).
The electrophilic synthesis of PAEK polymers
produces materials with reactive end groups such as
benzoic acids. Such polymers cannot be processed,
without endcapping, due to their high thermal
polyetherketone
(PEK)
became a possibility (
Scheme 2.1
)
[5]
.
Raychem also reported the synthesis of PAEK
polymers using similar reaction conditions in the
presence of alkylthiochloroformates.
Another electrophilic process exemplified by
Ueda and Oda uses methanesulfonic acid (MSA)/
phosphorus pentoxide (P
2
O
5
) at low temperatures
[6]
. Although PEEK produced by this method has
a less branched structure than AlCl
3
-catalyzed
systems, it also suffers from high temperature insta-
bility and hence cannot be molded or extruded
without extensive cross-linking and degradation.
Colquhoun and Lewis
[7]
have described the
Friedel Crafts polycondensation of 4-(4
0
-phenoxy-
phenoxybenzoic acid)
O
O
C
Cl
HF / BF
3
O
*
O
C
*
in trifluoromethanesulfonic
Scheme 2.1