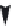Biomedical Engineering Reference
In-Depth Information
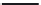
O
Scheme 2.2
O
O
C
OH
n
CF
3
SO
3
H
O
*
O
O
C
*
instability. When the reactive end group materials are
subjected to high-temperature processing, the poly-
mer immediately cross-links, producing gels, which
cannot be shaped into desired articles. Therefore,
PEEK production by electrophilic processes as
described earlier has historically had limited
commercial success.
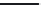
More recently, a modification to the electrophilic
process for manufacturing PAEK polymers has been
described. This again involves the polycondensation
of 4-(4
0
-phenoxyphenoxybenzoic acid). However,
methanesulfonic acid was used as the reaction
solvent in the absence of phosphorus pentoxide, and
1,4
0
-diphenoxybenzene was used as an endcapping
agent
[8]
. This route permits the manufacture of
thermally stable PAEK polymers and has been used
in industrial processes (
Scheme 2.3
).
It should be noted that to ensure thermal stability,
significant quantities of the endcapping agent are
used and as a result may be present in significant
quantities in the finished polymer. The choice of the
endcapping agent may therefore significantly alter
the leachable and biocompatibility profile of the
material.
2.2.2 Nucleophilic Routes to PAEK
Polymers
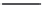
The nucleophilic route to PAEK polymers
provides a straightforward pathway to polymers such
as PEEK. Initial attempts to form high-molecular-
weight PAEKs from the reaction of a dihalobenzo-
phenone and an equivalent bisphenate failed due to
the polymer product crystallizing from the sulfolane
solvent (
Scheme 2.4
).
Owing to the poor solubility of PEEK, the selec-
tion of the synthesis solvent is crucial. Suitable
solvents should be thermally stable and inert to
phenoxide species. It became apparent that solvents
such as benzophenone or diphenylsulfone could be
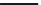
O
Scheme 2.3
O
C
OH
O
n
CH
3
SO
3
H
O
*
O
O
C
*
C
6
H
6
-O-C
6
H
6
End Capping Agent
O
O
C
O