Biomedical Engineering Reference
In-Depth Information
flow temperature (
T
f
). As we shall see, PEEK
components also exhibit a fourth transition, a recrys-
tallization transition (
T
c
), depending upon how it was
originally fabricated. In practical terms, all these
melt transitions occur at temperatures far exceeding
the boiling point of water and any clinical applica-
tions of the material. One of the characteristics of
PEEK is its high temperature stability, and it is used
in engine components for this reason. Although
tangential to the clinical function of the material,
some knowledge of its thermal behavior is crucial for
materials scientists and engineers who are interested
in producing PEEK implants, because thermal pro-
cessing is a critical step in the manufacturing of
PEEK components.
The glass transition (
T
g
) is classically considered
to be the temperature below which the polymer
chains are supposed to behave like a brittle glass.
Below
T
g
, the polymer chains have insufficient
thermal energy to slide past one another, and the
primary way for the material to respond to mechan-
ical stress is by stretching (or rupture) of the covalent
bonds constituting the molecular chain. With PEEK,
the glass transition occurs around 143
C. Ironically,
although PEEK is below the glass transition at room
and body temperatures, it is surprisingly ductile for
a “glassy” polymer, as it is capable of elongations of
up to 10
e
60%, depending upon the processing
method and testing conditions.
As we raise the temperature above
T
g
, the amor-
phous regions within the polymer gain increased
mobility, and secondary intermolecular forces (e.g.,
van der Waals forces) can influence the flow and
movement of the polymer chains. If the polymer
sample was quickly cooled down from the melt
during its previous history, when the temperature
rises above
T
g
, there will be a thermodynamic
tendency for the polymer to continue to form crys-
tals or to
recrystallize
. The features of this transition
provide clues to the materials scientist about how
the material was previously processed; however, for
implants, it has limited practical significance as the
component will remain below
T
g
for its entire
service life.
When the temperature of PEEK rises above its
recrystallization temperature, the smaller crystal-
lites in the polymer begin to melt. The melting
behavior of semicrystalline polymers, including
PEEK, is typically measured using differential
scanning calorimetry (DSC). DSC measures the
amount of heat needed to increase the temperature
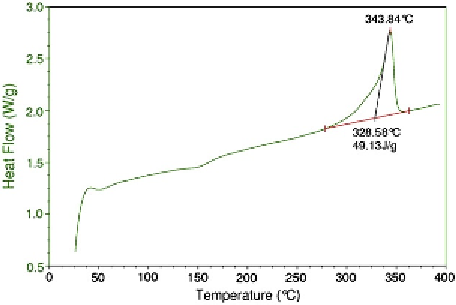
Figure 1.7
Representative DSC trace for PEEK
(annealed PEEK OPTIMA LT1 rod).
of a polymer sample. Some representative DSC data
for PEEK is shown in
Fig. 1.7
.
The DSC trace for PEEK shows several key
features. One feature of the DSC trace is its
recrystallization peak, which for this annealed rod
sample occurs around 150
C, corresponding to the
heat needed by the material to form crystals as it is
heated above the glassy state. The glass transition
temperature itself is difficult to discern from
a conventional DSC trace; a specially modulated
DSC analysis is usually needed to clearly demon-
strate the presence of
T
g
.
Another key feature of the DSC curve above
T
c
is the peak melting temperature (
T
m
), which for this
sample occurs at 343
C and corresponds to the
point at which the majority of the crystalline
regions have melted. The melt temperature reflects
the thickness of the crystals, as well as their
perfection. Thicker and more perfect PEEK crystals
will tend to melt at a higher temperature than
smaller crystals.
As the temperature of a semicrystalline polymer is
raised above the melt temperature (not shown on the
DSC trace), it may undergo a flow transition and
become liquid. PEEK undergoes a flow transition (
T
f
)
around 390
C and is typically processed at
this
temperature.
1.6 PEEK Composites
PEEK can be readily combined with certain
additives to create a
composite
. A composite
material is comprised of two or more distinct pha-
ses, each retaining unique physical, bioactive, and
mechanical properties, bonded together by an