Biomedical Engineering Reference
In-Depth Information
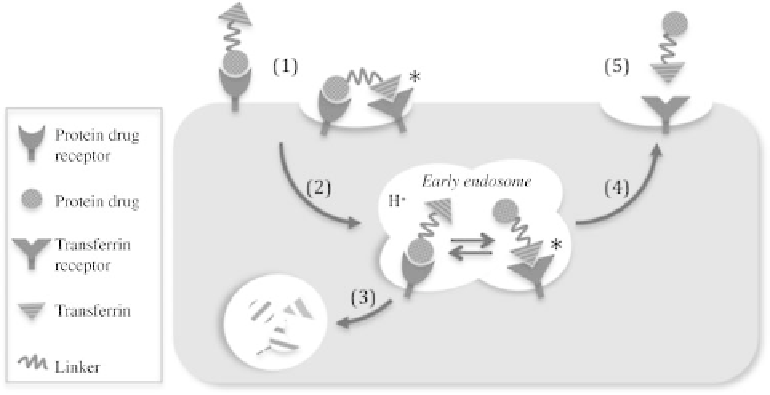
FIGURE 4.4
Endocytic pathway and intracellular metabolism of Tf-fusion proteins. In the
presence of abundant endogenous Tf, the Tf-fusion proteins first bind to protein drug receptor
(e.g., GHR or G-CSFR) on the target cell membrane via GH domain. This binding is the primary
binding, which enriches the fusion proteins onto the target cells. The protein drug receptor binding at
the cell surface brings Tf-fusion protein close to the plasma membrane surface, which may lead to
bivalent binding of the Tf-domain of the fusion protein to TfRs, which are present in the clathrin-
coated pit regions. This binding, indicated as “
” in the figure, is referred to secondary binding since it
occurs after the protein drug receptor binding [2]. The fusion proteins are endocytosed into the early
endosome, where TfR is also present [3]. Fusion proteins that remain bound to protein drug receptor
are degraded in the lysosome. With the acidification of endosome, the fusion proteins can dissociate
from protein drug receptor, and bind to TfR via their Tf domain, indicated as secondary binding “
.”
[4]. The binding to TfR allows the fusion protein to be recycled back to the cell surface [5]. The fusion
protein is released from TfR into the circulation at cell surface.
promotes the recycling of the fusion protein. The relative
receptor binding affinity of the two protein domains inside
the endosome will determine the impact of each receptor
binding on the plasma half-life of the fusion proteins.
This study highlights the importance of linkers in designing
and developing bifunctional fusion proteins as therapeutics.
First, the receptor binding affinities of the protein domains
could be altered via the insertion of linkerswith various lengths
and conformations. Second, it is feasible to fine-tune the PK
profiles of fusion proteins by linker insertion due to their
impact on the receptor binding and subsequent intracellular
processing. Third, this study also suggested that the different
functional domains in fusion proteinsmay play distinct roles in
determining the PK. Therefore, the impact of linker insertion
on each domain's function should be carefully evaluated to
achieve the desired PK profiles for the fusion proteins.
multidomain proteins may serve as a good lead for fusing
proteins of interest. In addition, many types of empirical
linkers such as flexible linkers, rigid linkers, or cleavable
linkers have been designed for various applications. An
optimal linker can provide many advantages for the fusion
proteins, including improving the structural stability,
improving bioactivity, increasing expression level, or alter-
ing the PK profiles of the fusion proteins.
When linker design is based on intuition, the outcome is
less predictable. Many exciting progress have been made
toward the rational design of linkers based on the require-
ments of target fusion proteins. To aid the rational design
of linkers, Crasto and Feng presented a program called
LINKER which can automatically generate a set of linker
sequences that are known to adopt extended conformations
as determined by X-ray crystallography and NMR [73].
The inputs include desired linker length, a number of
optional parameters such as name of protease, restriction
enzyme to eliminate sensitive sequence that may cause
instability of the linker. The program output contains a
group of linker sequences with a specified length. This
program could be a useful tool in facilitating the design of
optimal linkers.
4.5 CONCLUSIONS AND FUTURE PERSPECTIVE
During the development of recombinant fusion proteins as
therapeutics, linkers have become an indispensable compo-
nent
to achieve success. Linkers deriving from natural
Search WWH ::

Custom Search