Biomedical Engineering Reference
In-Depth Information
TABLE 5.3 High PBMC Response to FPX Peptides among Donors with High Anti-FPX Titers
aa 1-10
aa 11-20
aa 1-24
Subject ID
HLA-DRB1
Antibody Concentration (mg/mL)
IFN-
g
IL-4
IFN-
g
IL-4
IFN-
g
IL-4
1
0701/1501
20.2
1.8
0.8
26
89
34
92
2
0101/1301
1.5
1.5
1.8
9.9
4.7
26.6
30.1
3
0101/1701
1
0.6
1.4
14.6
6.8
16.9
14.5
4
0405/0101
1.1
1.4
1.2
6
4.7
9.4
7.1
of the in silico, in vitro, and clinical findings related to FPX1
and their concordance to each other, a similar risk assess-
ment strategy can be proposed for biotherapeutics under
early stage development.
5.6 PRECLINICAL AND CLINICAL
IMMUNOGENICITY ASSESSMENT STRATEGY
The assessment of immunogenicity of a biotherapeutic is
under considerable scrutiny by regulatory agencies. Hence,
as a part of risk minimization and mitigation, drug manu-
facturers should have a strategy to detect and characterize
the potential for immunogenicity. An evaluation of all
samples for binding antibodies in a screening assay is one
such strategy. The reactive samples are confirmed to contain
antibody using a secondary species-specific antibody. Addi-
tionally, a drug depletion step confirms the specificity of the
sample. The optimal platform for binding antibody assess-
ment is chosen based on the nature and modality of the
biotherapeutic. Lastly, a biological assay should be used to
test if these antibodies are capable of neutralizing the
biological effect of the drug [87,88]. The appropriate assays
when developed should be sensitive and specific enough to
eliminate false positive results. A fully characterized anti-
body response to a biotherapeutic enables risk assessment
and clinical relevance for the patient [89].
5.6.1 Strategy and Recommendation
A comprehensive approach to preclinical immunogenicity
testing could begin with a high throughput in silico screening
followed by an in vitro evaluation and end with testing in
vivo in transgenic animal models (Figure 5.4). Immunoge-
nicity screening could follow a tiered approach where Tier 1
would entail screening of linear sequences from multiple
therapeutic candidates for T-cell epitopes and clusters
therein. Candidates could then be ranked on the basis of
the quantity and quality of immunogenic epitopes, adjusted
for Tregitope content.
Once the field of candidates has been prioritized and/or
narrowed, Tier 2 screening would test the immunogenicity
of these molecules in one or more in vitro assays. At this
point, the in vitro assays would, in addition to validating
in silico predictions, bring forward any non-sequence-
related immunogenicity concerns such as processing-asso-
ciated changes, post-translational modifications and alter-
ations owing to misfolding. In vitro assays can also be
utilized to test therapeutics, which may have target-medi-
ated or agonist effects, and to overcome hurdles during
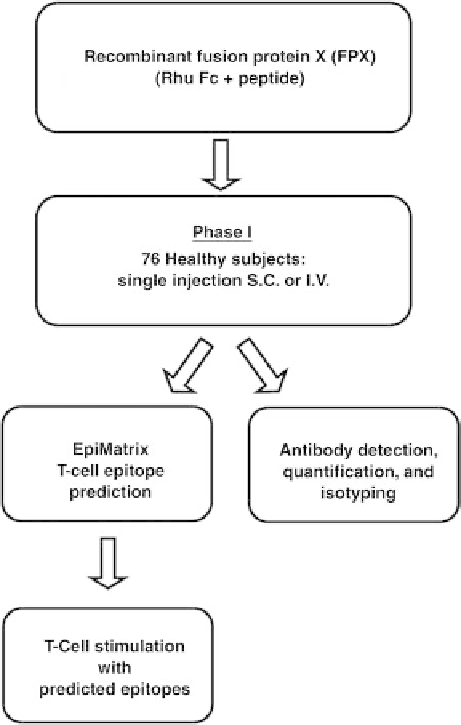
FIGURE 5.3
In silico prediction of immunogenicity—proof of
concept study in humans with FPX 1. In a prospective study,
sequences from four different fusion proteins were assessed
in silico for binding to HLA-DR alleles using the EpiMatrix
algorithm. High T-cell epitope content and low Tregitope content
were retrospectively linked with observed clinical immunogenicity
of FPX1 and followed by an evaluation of FPX1 immunogenicity
in vitro. On the basis of the in silico, in vitro, and clinical findings
related to FPX1 and their concordance to each other, a similar risk
assessment strategy may be proposed for biotherapeutics under
early stage development.












Search WWH ::

Custom Search