Biomedical Engineering Reference
In-Depth Information
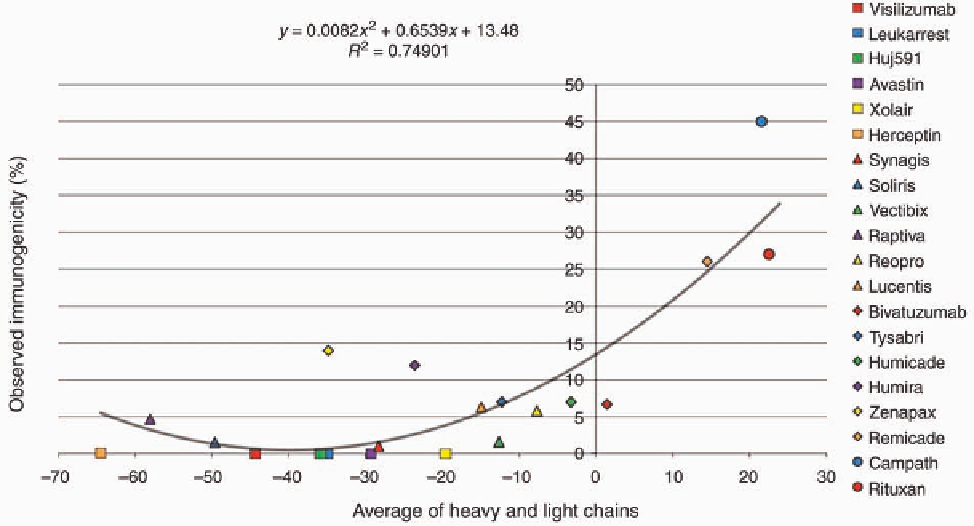
FIGURE 5.2
Predicted versus observed immunogenicity. Twenty human and chimeric antibodies
were selected from the literature based on the availability of in vivo immunogenicity data and
averaged where multiple studies of observed immunogenicity were available. Protein sequence
information for the variable regions of heavy and light chains was obtained (GenBank and the United
States Patent and Trademark Office). These sequences were analyzed with EpiMatrix and scored
according to the EpiMatrix immunogenicity scale. Observed immunogenicity was regressed against
predicted immunogenicity score of the combined heavy and light chains.
had a high rate of clinical immunogenicity, was associated
with elevated T-cell epitope content reflected by its high Z
score. Low Tregitope content was also associated with FPX1.
FPX2-4 was associated with a low EpiMatrix score, and
Tregitope adjustment reduced the predicted potential for
binding further. Accordingly, FPX2-4 showed only minor
clinical immunogenicity. Hence, an inverse relationship
between Tregitope content and immunogenicity rate could
be noted for these fusion proteins.
This retrospective study linking high T-cell epitope con-
tent and low Tregitope content with observed clinical immu-
nogenicity of FPX1 was followed by an evaluation of FPX1
immunogenicity in vitro by Koren et al. [52]. Whole blood-
derived PBMC were obtained from healthy individuals
dosed with FPX1. Cells were collected from both anti-
body-positive and antibody-negative subjects. Peptides,
derived from either the N-terminus (predicted as nonimmu-
nogenic by in silico algorithm) or the C-terminus (predicted
to be immunogenic by in silico algorithm) from FPX1, were
used for challenge in the recall response. Antigen-specific
responses from PBMC were assessed using IFN-
g
and IL-4
ELISpots as readouts. A significant induction of IL-4 and
IFN-
g
spot forming cells was observed in PBMC derived
from FPX1 antibody-positive subjects when the in vitro
cultures were stimulated with the C-terminus peptide as well
as the whole protein FPX1 (Table 5.3). Neither IL-4 nor IFN-
g
spot forming cells were observed in PBMC derived from
antibody-negative subjects and N-terminus peptide stimula-
tion in vitro. There was an excellent correlation between the
HLA restriction of the peptides, as predicted by EpiMatrix
algorithms, and the HLA of the patients who responded to
the epitopes [49].
The FPX1 fusion protein was further evaluated in a na
ıve
human PBMC-derived in vitro sensitization assay [54].
Whole PBMC were stimulated with FPX1-derived N-termi-
nus and C-terminus peptides multiple times to amplify the
frequency of antigen-specific cells. Immune responses were
measured by enumerating IFN-
g
-secreting cells by ELISpot.
A significant induction of IFN-
g
-secreting cells was evident
for the PBMC challenged with the immunogenic C-terminus
of FPX1 peptide. No reactive cells were observed when
challenged with nonimmunogenic N-terminus peptide and a
clinically proven and EpiMatrix-predicted nonimmunogenic
monoclonal antibody therapeutic.
This retrospective study validates the hypothesis that
immunogenicity of a biotherapeutic can be predicted
through the systematic application of in silico and in vitro
tools such as those described here (Figure 5.3). On the basis
€
Search WWH ::

Custom Search