Biomedical Engineering Reference
In-Depth Information
20.4.2 I
NJECTABLE
B
ULKING
M
ATERIALS
Injectable bulking agents constitute a feasible and attractive procedure to replace conventional
surgery for a number of pathological conditions. Bulking agents have been used for many years
to successfully treat patients with urinary incontinence [24]. As a result of this success, a small
number of pilot studies have recently reported the use of bulking materials for the treatment of fecal
incontinence in patients with weak but intact internal anal sphincter.
20.4.2.1 Polymer Microspheres
The fi rst reported injectable bulking biomaterial used in the treatment of partial fecal incontinence
was a paste of polytetrafl uoroethylene (Tefl on [Du Pont de Nemours & Company, Inc., Wilmington,
Delaware] or Polytef), one of the most widely used biomaterials in medicine [25]. Polytef paste is
a mixture of polytetrafl uoroethylene, glycerin, and polysorbide fabricated as microspheres with
sizes ranging from 4 to 100 µm, with 90% in the 4-40 µm range. Despite the reported therapeutic
effi cacy of Polytef, polytetrafl uoroethylene-based materials have many drawbacks that limit their
usefulness for sphincter augmentation. Moreover, polytetrafl uoroethylene spheres have been found
to migrate from the implantation site to distant organs and because they are nonbiodegradable are
thought to have triggered granulomatous foreign body reactions, fever, and pneumonitis [26,27].
The problem of detecting transmigration of bulking biomaterials is exacerbated by materials, such
as poly(methyl methacrylate), polytetrafl uoroethylene, and silicone rubbers being radiolucent, that
is, invisible with x-ray imaging. Because of the potentially serious clinical implications of this type
of material, an ideal long-lasting injectable biomaterial is sought as a bulking agent for incontinence
that is nonantigenic, volume-stable, and nonmigratory.
Microspheres remain an attractive choice for bulking materials due to their relative ease of
delivery and ability to conform to the shape of the implantation site. A number of fabrication tech-
niques for producing microspheres have been developed and reported to date, but the choice of the
fabrication technique depends on the nature of the polymer, the intended use, and the duration of
the therapy. Whichever technique is used, the following criteria should be achievable: (i) the yield
of microspheres within the desired size range should be high, (ii) the quality of microspheres should
be reproducible, and (iii) microspheres produced should be free fl owing and should not aggregate
or adhere to one another.
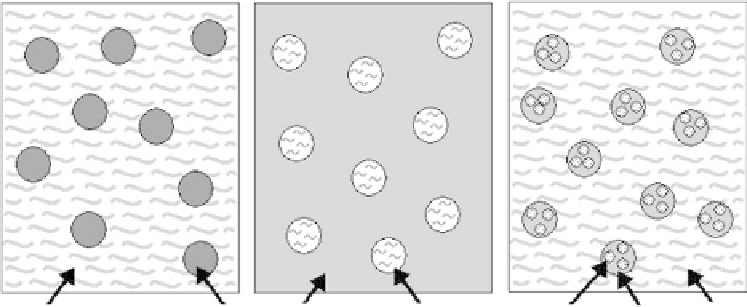
Emulsion solvent diffusion techniques are frequently used to produce microspheres and can be
classifi ed as either “in-water” methods or “in-oil” methods (Figure 20.3). The oil-in-water single
Oil-in-water
Water-in-oil
Water-in-oil-in-water
2
1
Water
Water
Oil
Oil
Water
Water
Oil
FIGURE 20.3
Schematic illustration of single and double emulsion processes for the fabrication of
microspheres.