Biomedical Engineering Reference
In-Depth Information
with defi nable micro- and nanostructures provides an opportunity to develop enhanced biological
and medical devices, which may lead to enhanced performance of devices such as vascular grafts,
tissue scaffolds, and templates for guided tissue regeneration. There are two differences between the
CES and the FFESS processes. Firstly, the FFESS relied on a charge-injection electrode to charge
the solution surface and resulted in reproducible charging and stable axisymmetric electrospraying.
Secondly, at high voltages the charge injection was dominated by fi eld injection because of the
sharp electrode, and thus generated fi ner particles and fi bers.
11.2.8.2.3
Native Biomaterials
The manufacturing of protein and other biomolecule biomaterial fi lms onto an electrode surface is a
necessary step in the fabrication of enzyme electrodes and other types of biosensors for biomedical
application. Morozov and Morozova were the fi rst to prepare functionally active protein fi lms
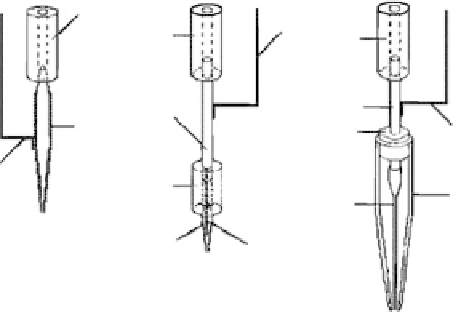
using electrospraying [77]. A schematic diagram of the setup for depositing protein is shown in
Figure 11.27. They used a solution prepared by dissolving a commercial dry powder of alkaline
phosphates in water. In the experiment, three different designs of the capillaries for electrospraying
were studied. The fi rst design, as shown in Figure 11.27a, was a glass capillary coated with a silver
layer on the external surface of a glass capillary. A conductive wire that is in contact with the coated
capillary was connected with a high-voltage power supply. In the second design, as shown in Figure
11.27b, the metal electrode was not exposed to a gas phase in order to reduce the risk of corona
discharge at high voltage, which was a glass capillary with an inner electrode. In the third version
of the capillary design, as shown in Figure 11.27c, a liquid bridge between the electrode and the
solution was introduced to avoid any contact of the metal electrode with the protein solution. The
external surface of the stainless steel tube was used as an electrode exposed to the interior of the
large external glass capillary, whereas the internal thin plastic capillary was used to supply protein
solution. The setup for depositing protein materials is shown in Figure 11.28. The experimental
results showed that the functional activity of alkaline phosphates could completely survive the
electrospraying process. Electrospraying neither destroyed the compact native structure of alkaline
phosphates protein molecules nor irreversibly inactivated them. It is defi nitely a feasible technique
to fabricate biologically specifi c fi lm materials for biosensors, libraries, and diagnostic assays.
(a)
(b)
(c)
1
3
1
1
4
4
2
1
3
3
1
5
7
5
6
FIGURE 11.27
Types of capillaries used for electrospraying: (a) capillary with external electrode, (b)
capillary with internal electrode, and (c) capillary with a liquid bridge. (1) Plastic tubing, (2) glass capillary
coated with the silver layer, (3) contact wire, (4) stainless steel tube, (5) glass capillary, (6) internal tung-
sten or stainless steel electrode, and (7) plastic capillary. (Reprinted from Morozov, V.N. and Morozova,
T.Y.,
Anal. Chem.
, 71, 1415, 1999. © American Chemical Society. With permission.)