Biology Reference
In-Depth Information
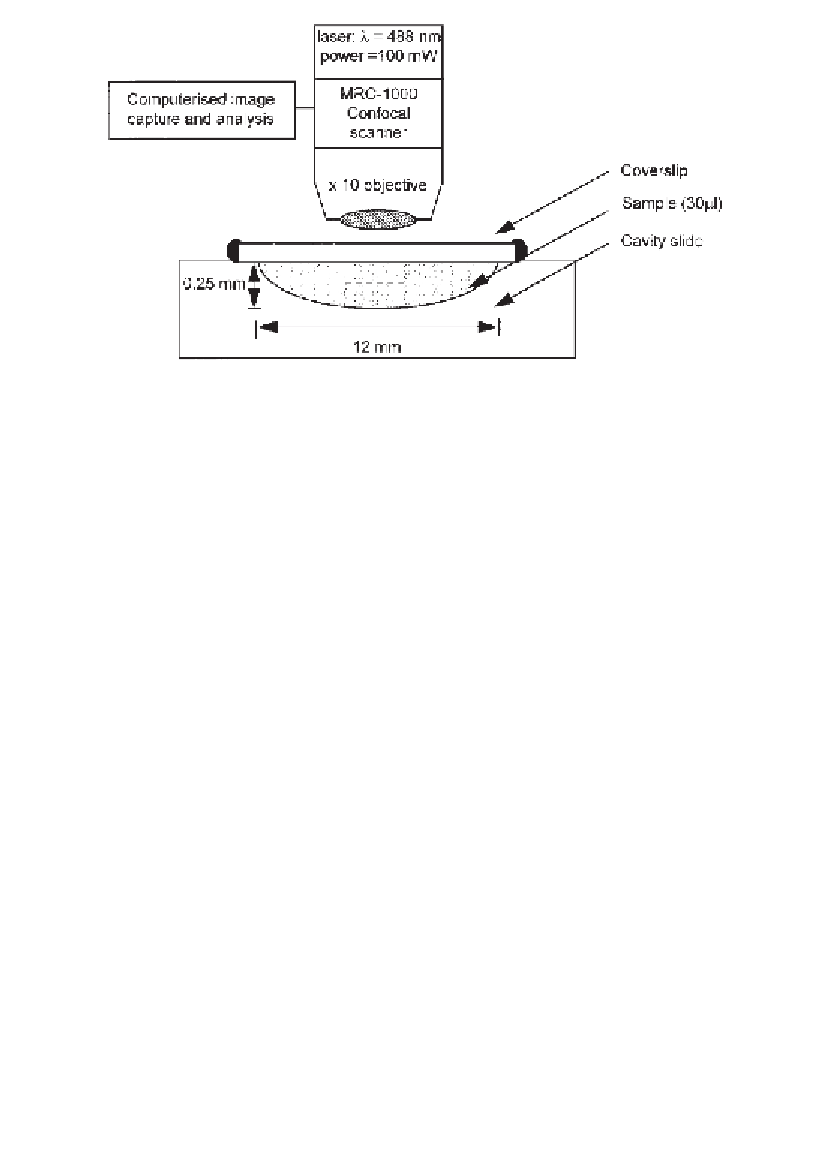
L) is contained
in a cavity microscope slide with a sealed coverslip. Square bleaches are generated by
scanning at high laser power through the volume of the sample. Fluorescence recovery
is observed at low laser power in a focal plane (- - - - ) in the centre of the slide,
midway between coverslip and slide.
Fig. 3. Experimental arrangement for confocal FRAP. Sample (30
µ
vary greatly. The pharmaceutical grade material is least contaminated (<0.1 %
protein). For tracer experiments, commercial protein preparations sold as
“molecular weight standards” can usually be fluorescein-isothiocyanate (FITC)
labeled and used without further purification (e.g., soy bean trypsin inhibitor
and bovine serum albumin from Sigma). However, many commercially avail-
able FITC-labeled proteins are excessively substituted (>10 mol FITC per mol
protein) and should be avoided as their diffusion properties may differ signifi-
cantly from those of the native protein. FITC-dextrans are useful, uncharged,
matrix probes and are available in many different sizes from molecular weight
4 kDa to 2500 kDa (Sigma). At present, commercial sources of aggrecan are
expensive, but it can be prepared by extraction of cartilage and stored dry or in
solution at -20
C
(19)
. The labeling protocols outlined for proteins and carbo-
hydrates such as hyaluronan are generally applicable, although for molecules,
such as aggrecan, the protein content is only ~10% by weight, so correspond-
ingly reduced amounts of FITC are sufficient for labeling. Concentrated solu-
tions of aggrecan and HA are viscous, so care should be taken to minimize
handling losses.
°
3.1.1. Protein Labeling
1.
Dissolve 10 mg of protein in 5 mL of carbonate labeling buffer (pH 9.0) by mix-
ing with gentle rotation for 24 h at 4
°
C (48 h for aggrecan solutions).
Search WWH ::

Custom Search