Biology Reference
In-Depth Information
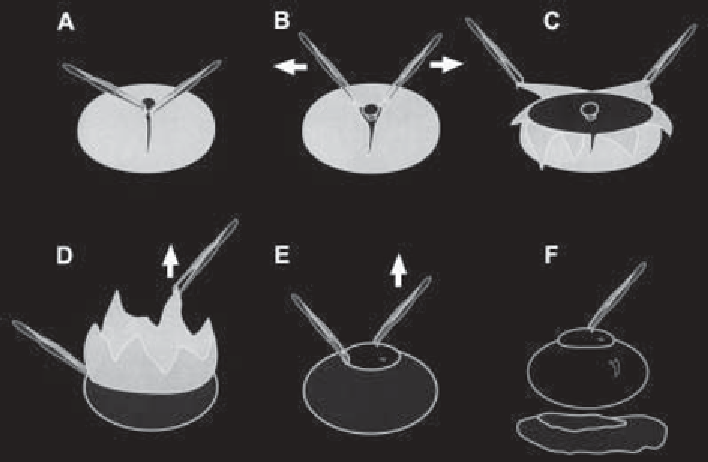
Fig. 1. Dissection of the chick neuroretina (tissue depicted in grey) from the pig-
mented epithelium (black) and lens/vitreous body (white).
See text
for details.
5.
A volume of the cell suspension is diluted in DMEM/F12 Ham (Sigma) medium
containing N2 supplement to reach 275,000 cells/mL (after a complete cellular
dissociation, around 100
µ
L of the suspension should be added to 900
µ
L of
medium; never dilute the medium below 50%).
6.
Remove the substratum solution from the four-well Petri dishes described in the
last section and wash three times with Ca
2+
-, Mg
2+
-free PBS. Then, remove this
buffer, transfer 55
C in a
water-saturated atmosphere containing 5% CO2. Most cells will attach to either
substratum within 20 min of plating. Finally, add 2 mL of previously prewarmed
DMEM/F12 Ham medium containing N2 supplement and 0.5
µ
L of the cell suspension and incubate for 1 h at 38
°
Ci/mL [
3
H]methyl
µ
thymidine and incubate the same conditions for 20 h.
7.
After 20 h in vitro, cells are immunolabeled with a neuronal marker (G4). First,
the coverslips are picked up with fine forceps and washed three times with KRH.
Then they are put in new four-well Petri dishes and incubated for 20 min in 1:100
dilution of G4 mAb ascitic fluid in KRH at room temperature. After this incuba-
tion time, coverslips are rinsed again three times with KRH and incubated with
biotinylated goat antimouse antibodies diluted 1:100 in KRH for 20 min at room
temperature. Finally, the coverslips are rinsed again three times with KRH and
incubated in Streptavidin-Texas Red (1:100) in KRH for 20 min at room tem-
perature. After this incubation, the coverslips are rinsed in KRH three times and
then the cells are fixed by incubation in 4% paraformaldehyde for 30 min.

Search WWH ::

Custom Search