Biology Reference
In-Depth Information
expected by Watson-Crick base pairing.
23
Our earlier SAXS data clearly
showed that the ribozyme can be monomeric in solution
46
(and indeed a
monomer taken from the crystal structure fits with the electron density
envelope calculated from SAXS data) so that it is probable that there is a
monomer-dimer equilibrium in solution.
The active site of the dimeric ribozyme is clearly seen in the crystal. G620
and A621 flanking the scissile phosphate are splayed apart and are stacked
with G638 and A756, respectively. Thus, these nucleobases are positioned
consistent with their proposed roles as general base and acid, respectively, in
the cleavage reaction. For this, they would need to move a little closer to
form the active state, but there is nothing that would seem to prevent this.
Thus the crystal structure is in excellent agreement with our earlier mech-
anistic studies.
2.6. The catalytic mechanism of the VS ribozyme
There are a number of possible roles for A756 and G638 in the generation of
the catalytic rate enhancement. They might stabilize the transition state by
hydrogen bonding the phosphorane, or a positively-charged protonated
adenine base might provide electrostatic stabilization of the dianionic tran-
sition state. However, the available evidence points toward general acid-
base catalysis by these nucleobases.
A chemical mechanism consistent with the roles of G638 and A756 in
general acid-base catalysis is shown in
Fig. 3.5
.
22
Clearly, this catalytic
A
B
H
2
N
N
N
GUA
GUA
N
N
O
O
N
N
N
N
O
O
H
O
O
H
H
2
N
O
P
O
P
O
O
O
O
O
ADE
ADE
O
O
H
H
N
N
N
N
O
OH
O
OH
O
NH
2
N
N
N
N
Figure 3.5 Two alternative mechanisms for cleavage of the hairpin and VS ribozymes
based on general acid
-
base catalysis by adenine (A38 and A756, respectively) and gua-
nine (G8 and G638) nucleobases. (A) Deprotonated guanine acts as general base to
deprotonate the 2
0
-O, and protonated adenine protonates the 5
0
-O leaving group.
(B) Neutral adenine acts as general base to deprotonate the 2
0
-O, and neutral guanine
protonates the 5
0
-O leaving group.
































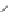




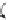













































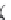










Search WWH ::

Custom Search