Biomedical Engineering Reference
In-Depth Information
develop transgenic plants (genetically modified plants) that produce PHAs (Mittendorf
et al
.,
1999; Snell and Peoples, 2002). Presently, a variety of PHAs can be synthesized in many
transgenic plants such as tobacco, rapeseed, cotton, alfalfa, flax, and oil palm (Yunus
et al
., 2008 ).
Moreover, alternative carbon sources for bacterial fermentations to synthesize PHAs are
also under investigation. These include fatty acids (Tan
et al
., 1997 ; Chakraborty
et al
.,
2009) and glycerol (Ibrahim and Steinbüchel, 2009). With the aid of genetically-modified
bacterial strains a direct utilization of plant oils, for example bacteria with lipase activity to
utilize triacylglycerols, has also been described (Marsudi
et al
., 19988). Furthermore, it was
shown that by varying the fatty acid feed composition, the properties of the plastic material
can be tuned (Lemos
et al
., 2006). Additionally, low-grade coal liquefaction products could
also be used as carbon source, since low-grade coal exists in high amounts and has no value
for common industrial processes (Füchtenbusch and Steinbüchel, 1999).
11.2.5 Polymers from chitin or chitosan
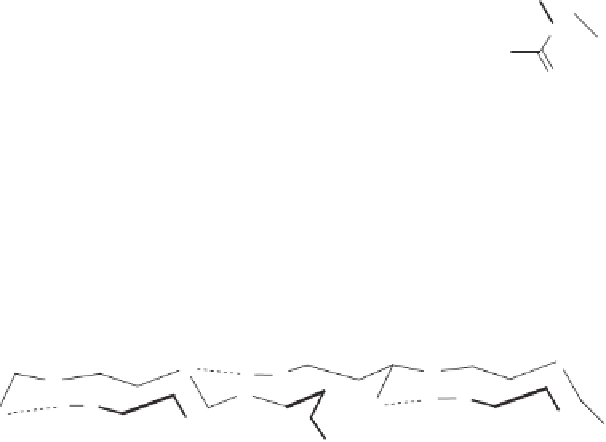
Chitin is a polysaccharide found in the outer skeleton of crustaceans, insects, crabs, shrimp,
lobsters, in the internal structures of other invertebrates, and in the cell walls of fungi. Chitin
(poly-N-acetyl-d-glucosamine, 2-acetamido-2-deoxy-1,4-
-d-glucan) (Figure 11.6 ) is one
of the three most abundant polysaccharides in nature, is biodegradable, non-toxic, and
readily biocompatible.
Chitosan is also a natural product and can be obtained
via
(partial) deacetylation of chitin,
most commonly by aqueous alkali, although this N-deacetylation is almost never complete
β
O
O
OH
OH
NH
NH
O
O
H
O
O
O
HO
O
H
O
O
HO
H
O
O
NH
NH
OH
n
OH
OH
O
O
Chitin
Partial deacetylation
OH
OH
NH
2
NH
2
O
O
O
O
H
O
HO
HO
O
H
O
O
H
O
O
NH
2
NH
2
n
OH
OH
OH
Chitosan
Figure 11.6
Chemical structures of chitin and chitosan.










Search WWH ::

Custom Search