Biomedical Engineering Reference
In-Depth Information
resulting in a more hydrophilic and highly crystalline polymer. Interestingly, no linear
relationship between the composition and degradability was reported; hence, copolymers
with high or low comonomer ratios are less sensitive to hydrolysis than copolymers with
equal ratio, due to their greater crystallinity (Reed and Gilding, 1981). Another medical
application of PLA and its copolymers, owing to their tissue compatibility, is the preparation
of scaffolds for tissue engineering (Kayaman-Apohan
et al
., 2001 ).
11.2.4 Polyhydroxyalkanoates
Polyhydroxyalkanoates (PHA) are a form of carbon and energy storage in the cytoplasm
of most of the bacteria belonging to the Halobacteriaceae family (Steinbüchel and
Füchtenbusch, 1998 ; Flieger
et al
., 2003). After the destruction of the cell walls, up to 90%
poly(3-hydroxybutyrate) (PHB) polymer, the most known class of PHAs, can be isolated
(Mecking, 2004). These polymers are biodegraded by microbes within five to six weeks.
During this period they can be metabolized, both in the presence and absence of oxygen,
into carbon dioxide and water or methane, respectively (Shimao, 2001).
PHAs can be used for the manufacture of films, coated paper, compost bags and can also
be molded into bottles and razors (Mooney, 2009). Additionally, since they are biocompatible,
they can also be used as implants without causing inflammation (Ramakrishna
et al
., 2001 ).
Copolymers of PHAs are more useful for the industry, since they exhibit lower crytallinity,
easy processability, and more flexible final product properties. The final PHA polymer
properties can thus be adjusted from semi-crystalline to elastomeric plastics. The most
common instance is poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV). The relative
content of hydroxybutyrate and hydroxyvalerate monomers can be adjusted by the amount
of the valine added to the glucose-based culture medium (Eschenlauer
et al
., 1996 ). The
melting point of PHB (180 °C) is lowered to 137 °C by 25 mol% of hydroxyvalerate units
(Mecking, 2004). PHBV was first commercialized by ICI (Imperial Chemical Industries plc)
in the late 1980s as Biopol
®
and its production was stopped in 1998 after it was sold to
Monsanto in 1996. Currently, the Biomer Company in Münich, Germany, produces about
100 kg of PHB per cubic meter of fermentation medium; a white powder with 98% purity
can be collected after extraction (Figure 11.5).
PHAs require expensive bacterial fermentation and isolation processes resulting in more
expensive production costs if compared to other petroleum-derived polymers. Thus, in the
beginning of the 2000s, an alternative strategy for lowering the production costs proposed to
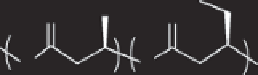
O
*
*
O
O
n
PHB
1. Fermentation
or
Glucose
2. Extraction
Precipitation
O
O
*
O
O
n
m*
PHBV
Figure 11.5
Synthesis of PHAs.






Search WWH ::

Custom Search