Biomedical Engineering Reference
In-Depth Information
PEG would suppress hydrophobic interactions between chitosan chains, thereby resulting
in a solution that does not gel at body temperature. Thermoreversible chitosan/PEG hydro-
gels are obtained when PEG is grafted in an amount of 45-55 wt% [35]. Similar trends
were observed by Ganji and Abdekhodaie [36] for thermosensitive chitosan/PEG block
copolymers. Harding and coworkers [37] found that the swelling ratio of cross-linked
chitosan-g-PEG hydrogels increases with increasing temperature. And the chitosan/
PEG semi-IPN also exhibits similar swelling behaviors [38].
5.3.1.3 Chitosan/PVA Thermosensitive Hydrogels
PVA, a water-soluble polyhydroxy polymer, has been frequently explored as implant mate-
rial for drug delivery systems and surgical repairs because of its excellent mechanical
strength, biocompatibility and nontoxicity. The formation of chitosan/PVA hydrogels
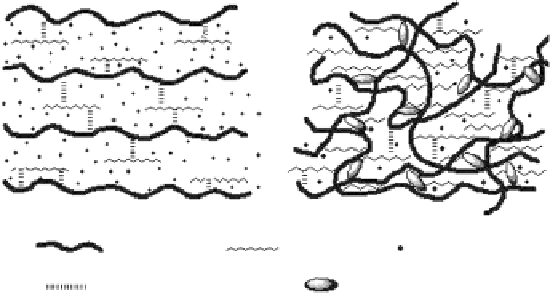
could be controlled by temperature. The chitosan/PVA composites are liquid solutions at
low temperature (about 4°C), but gel under physiological conditions [39]. At low tempera-
ture, it is very difficult to construct hydrogel networks using chitosan and PVA because of
the difficulty of creating contacts between the junction chains. In this condition, hydrogen
bonds exist not only between the OH and NH
2
groups of chitosan and the OH group of
PVA but also between PVA and water due to the high hydrophilicity of PVA, which can
lead to the dissolution of chitosan chains. In addition, the low temperature can also reduce
the mobility of chitosan molecules, which further prevents the association of chitosan
chains (
cf.
Figure 5.15a). However, the high temperature can reduce the intermolecular
hydrogen-bonding interactions and accelerate the mobility of chitosan molecules. So the
energized water molecules surrounding the chitosan chains are removed. The dewatered
hydrophobic chitosan chains associate with each other (
cf.
Figure 5.15b) [40]. That is to say,
chitosan is responsible for the hydrophobic interactions at high temperature, while PVA
content is related to the hydrogen-bonding interactions at low temperature. Therefore, the
proportion of PVA in the gel increases, gelation time becomes longer at 37°C, the aperture
turns smaller, and hence the intensity of the gel increases. When the ratio of PVA to chito-
san is greater than 10:1, chitosan/PVA would not be thermosensitive; hence, the content of
PVA should be kept at a certain degree [41].
(a)
(b)
: Chitosan
: PVA
: Water
: Hydrogen bonds
: Hydrophobic interaction
Figure 5.15
Formation mechanism of chitosan/PVA gel: (a) solution at low temperature and (b) gel at high temperature.
Search WWH ::

Custom Search