Biomedical Engineering Reference
In-Depth Information
obviously showed a different scattering behavior for the approaching
of H
with different incident energies [10], confirming the expected
graphite edge reactivity.
2
5.4
Hydrogen Clustering on Graphene
On the experimental side, Zecho
[11] characterized graphite
basal-plane adsorbed hydrogen, verifying by electron energy loss
spectroscopy (EELS) a reasonable agreement with the density
functional theory (DFT) calculations. Prominent peaks from
thermal desorption spectroscopy (TDS) measurements have been
furthermore confirmed to be the contributions of the deuterium (D)
atoms adsorbed on the terraces [12]. Analyses of the spectra through
numerical simulations of the first order desorption kinetics, from
separate TDS profile contributions, suggest the presence of relatively
isolated adsorbed deuterium atoms, close-pairing deuterium dimers,
and mixtures of these on the graphite surface. Very recent scanning
tunneling microscopy (STM) measurements [13, 14] confirm it, in
particular showing four distinct pair configurations on graphene. In
addition to the presence of pairs, adsorbed clusters of four hydrogen
atoms ordered along the perimeter of a graphite hexagon have
furthermore been proposed from a comparison of high-resolution
EELS with simulated vibrational spectra [15].
et al.
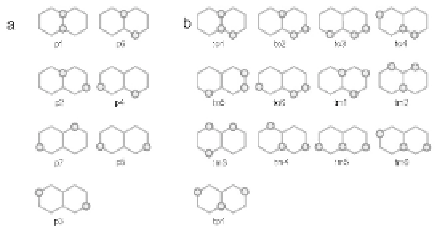
Figure 5.5
Closest packing hydrogen clusters, chosen by the fact that H
atoms favor a top-site adsorption: (a) pairs/dimers, and (b)
trimers adsorbed on one face of graphene.
Understanding the stability of small groups of hydrogen atoms
adsorbed on graphene is fundamental for tracing the succeeding
steps in order to achieve the saturation levels. It is in this context