Biomedical Engineering Reference
In-Depth Information
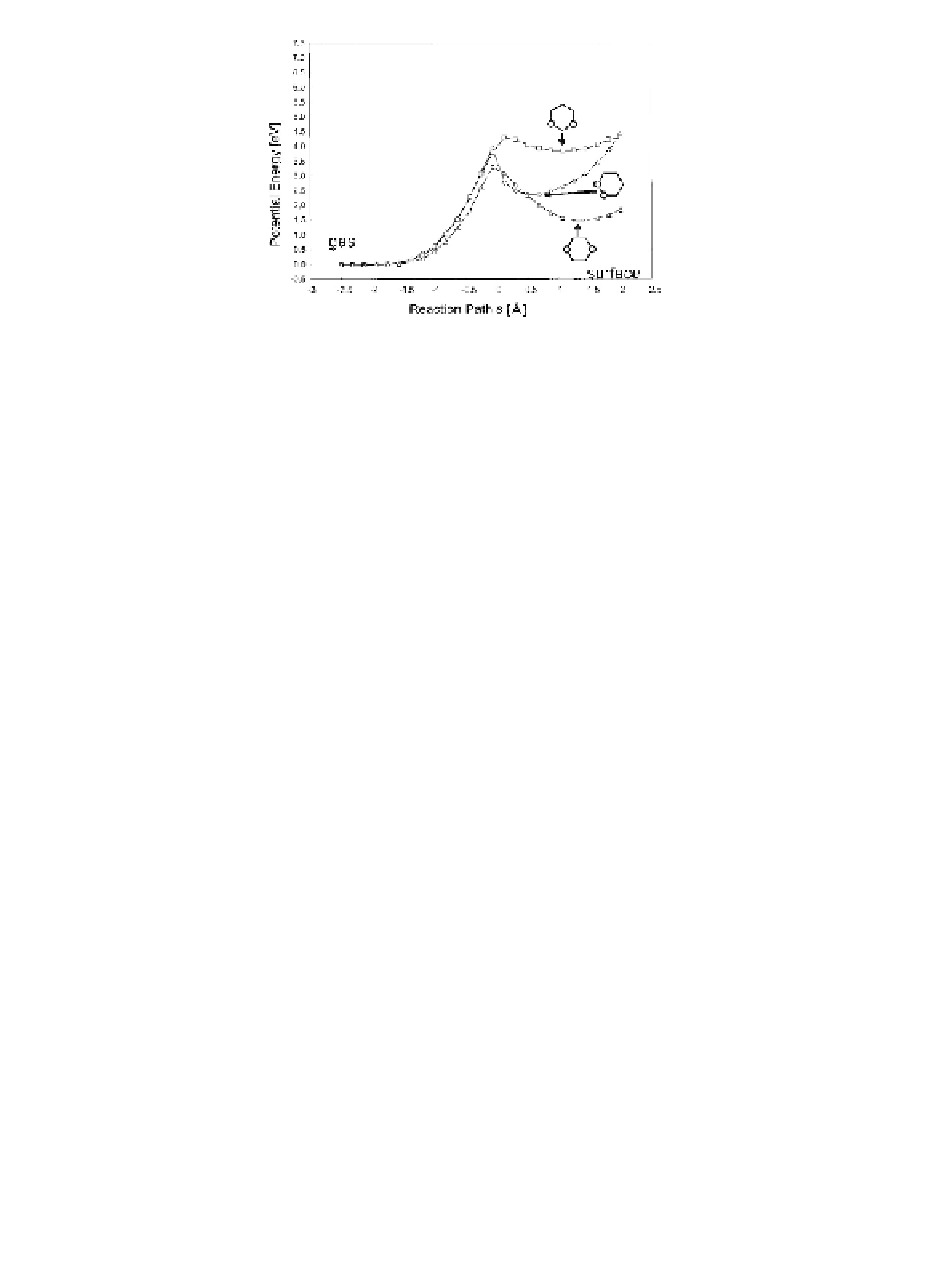
Figure 5.4
Reaction paths for H
dissociative adsorption on graphene;
hydrogen surface coverage of 1/4. Carbon atoms were allowed
to relax in these calculations. (Adopted from Ref. [7].)
2
Ideal graphene is the infinite planar arrangement of carbon
atoms. Up to this point we have been discussing the adsorption of
hydrogen on a graphene face. However, when we deal with carbon
nanomaterials, edge sites become extremely significant with respect
to hydrogen reactions, such as in the H attachment onto the edge of
a graphene nanoflake (Fig. 5.1). Edge defects show a vastly different
behavior from the graphene surface carbon atoms because of the
interplay between the energy released in passivating dangling
bonds defect at the edges and the abovementioned energy needed
to dissociate the strongly bound H
molecule. Previous studies in our
research group have been specifically carried out on the interaction
of hydrogen molecules with bilayer graphene edge defects [8, 9],
showing very strong adsorption values: -4.5 eV on the armchair
edge, and -4.72 eV on the zigzag edge (-4.5 eV and -4.6 eV for
atomic H energy reference respectively; the negative values indicate
that adsorption is energetically favored).
Comparing these values with the graphene surface-adsorbed
hydrogen, the adsorption energy per H atom is definitely much
higher, and hence desorption for edge-adsorbed hydrogen atoms
is a very difficult process. Furthermore, it has been shown that the
zigzag edges are more reactive towards molecular hydrogen as
compared with the armchair edge. H
2
dissociative adsorption on the
zigzag edges has no barriers, while the one on armchair edge has to
overcome a barrier of at least 0.3 eV. Quantum dynamics calculations
on the obtained potential energy surfaces on these two distinct edges
2