Biomedical Engineering Reference
In-Depth Information
For clarity, only the samples activated at 1000°C are plotted
in Fig. 3.9. As expected, the total micropore volume of CAC-A1000
is larger than that of CA-A1000. The total micropore volume is
1.06 cm
/g for CA-A1000,
respectively. There is an increase in micropore volume when
the sample was activated. Moreover, a larger micropore volume
(0.34 cm
3
/g for CAC-A1000 and 0.75 cm
3
3
/g) for CAC was obtained when compared to a sample
from [25] with a similar surface area of 680 cm
2
/g. It is suggested
that the base catalyst KOH is beneficial to the formation of
micropores, which is a practical method to increase hydrogen
storage capacity in CAs. Moreover, for the catalyzed samples, the
heat treatment at high temperatures produced an increase in
cumulative pore volume and a shift of the PSD to pores of larger
size. The detailed results are listed in Table 3.4.
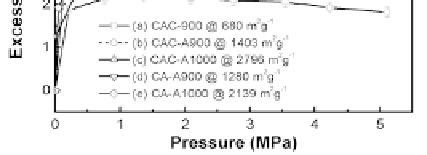
Figure 3.10
Hydrogen adsorption isotherms at 77 K for catalyzed CA
(a, b, c) and uncatalyzed CA (d, e). The unactivated sample is
for (a), the activated temperatures are 900°C for (b) and (d),
and 1000°C for (c) and (e) [49].
adsorption isotherms on various CA.
Enhanced hydrogen storage capacities were found in the KOH
catalyzed samples; the maximum values are 5.2 wt.% for CAC-A1000
at 3.5 MPa and 4.2 wt.% for CA-A1000 at 2.7 MPa, 3.1 wt.% for CAC-
A900 at 2.8 MPa and 2.9 wt.% for CA-A900 2.7 MPa, respectively (see
Table 3.4.). For comparison, the maximum hydrogen uptake of CAC
is only 2.2 wt.%, which is due to the lowest surface area of 680 m
Figure 3.10 presents H
2
2
/g.
The H
uptake capacity is 5.2 wt.% for CAC-A1000 at 2.5 MPa, which
2