Biomedical Engineering Reference
In-Depth Information
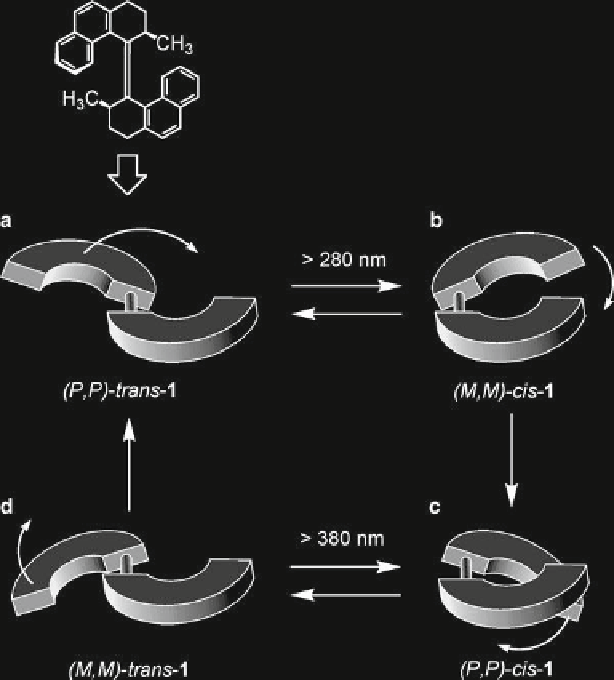
Fig. 3
Light-driven unidirectional rotation of the two helical subunits of compound
1
. Reproduced
by permission from Balzani et al.
2003
(Fig.
3c
). Subsequent irradiation (³280 nm) of the solution produces a mixture of
(
P
,
P
)-
cis
-
1
and (
M
,
M
)-
trans
-
1
in a ratio of 10:90 (Fig.
3d
). Upon increasing the
temperature further (333 K), (
M
,
M
)-
trans
-
1
interconverts irreversibly to the original
isomer (
P
,
P
)-
trans
-
1
. Thus, a sequence of light- and heat-induced isomerizations
can be exploited to move this molecular rotor in one direction only. Indeed, when
(
P
,
P
)-
trans
-
1
is irradiated (³280 nm) at a high temperature (293 K), a clockwise
360° rotation occurs and therefore the motor exhibits autonomous behavior. The
overall process can be followed by monitoring the changes in the circular dichroism
spectra. The unidirectional motion in this system is dictated by the stereogenic cen-
ters associated with the two methyl substituents. More recently, this molecular
motor was redesigned to improve its performance, (Ter Wiel et al.
2003
) and the
structure was modifi ed so that the motor can be powered by visible instead of UV
light (Van Delden et al.
2003
). Furthermore, it was shown that unidirectional rota-
tion can be controlled by a single stereogenic center (Koumura et al.
2000
) .