Biomedical Engineering Reference
In-Depth Information
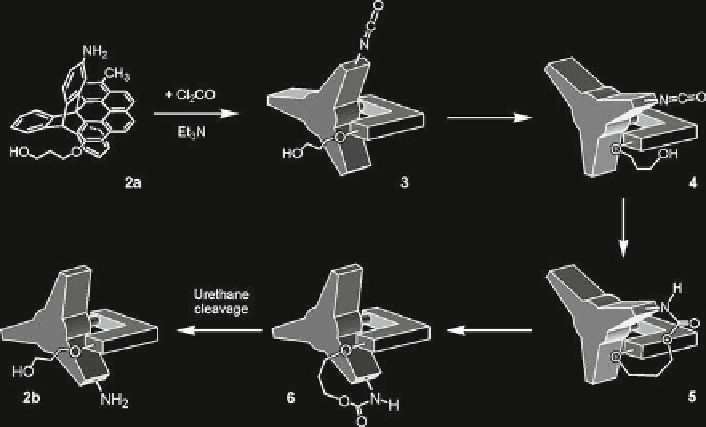
Fig. 4
Sequence of events causing unidirectional rotation of 120° in a triptycene/helicene system
powered by phosgene as a chemical fuel. Adapted by permission from Balzani et al.
2003
3.1.2
Toward a Chemically Driven Rotary Motor
An important step toward the realization of a chemically powered molecular motor is
the unidirectional rotation illustrated in Fig.
4
(Kelly et al.
1999,
2000
) . Compounds
2a
and
2b
are two of the three low-energy rotamers about the axle connecting the
triptycene and helicene components. Rotamer
2a
is activated by reaction with phos-
gene to give isocyanate
3
, which is chemically “armed” to react with the OH group in
the hydroxypropyl tether attached to helicene. However, in the rotational ground state
3
,
the isocyanate and the OH group are too far apart to interact. However, at those instants
when a clockwise rotation of the triptycene (not possible with a comparable counter-
clockwise rotation) brings the isocyanate and the OH group suffi ciently close to react
(see
4
), urethane formation can then result (
5
), irreversibly trapping the triptycene in
a relatively high energy conformation around the triptycene/helicene axle. Ambient
thermal energy then drives the exergonic, but very slow, unidirectional rotation from
5
to
6
. Finally,
6
is cleaved to
2b
, thereby completing the chemically driven rotation
of
2a
to
2b
. Admittedly, after this proof of principle, much work has still to be done to
obtain a system that can undergo a full, continuous, and fast rotation.
3.1.3
Other Systems
It was shown (Raehm et al.
1999
; Kern et al.
2000
; Poleschak et al.
2004
) that in suit-
ably designed rotaxanes the pirouetting-type movements of the ring around the axle
can be electrochemically driven. Similarly, controlled pirouetting of the interlocked