Biomedical Engineering Reference
In-Depth Information
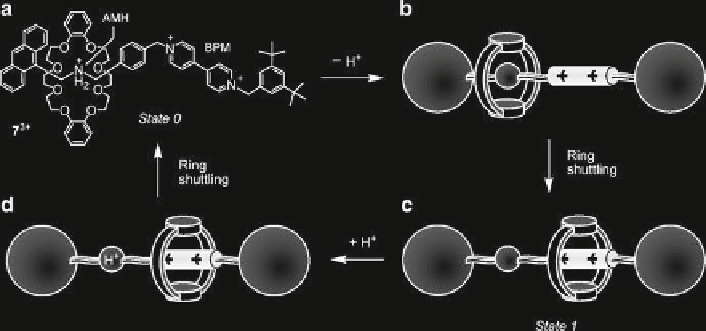
Fig. 5
Schematic representation of the operation of the acid/base-controllable molecular shuttle
7
3+
. Adapted by permission from Balzani et al.
2003
onto the ammonium center. Such a switching process was investigated in solution
by nuclear magnetic resonance (NMR) spectroscopy and by electrochemical and
photophysical measurements (Ashton et al.
1998
). Recently, the kinetics of ring
shuttling were also studied in detail by stopped-fl ow spectroscopic experiments
(Garaudée et al.
2008
). The full chemical reversibility of the energy supplying acid/
base reactions guarantees the reversibility of the mechanical movement, in spite of
the formation of waste products. Notice that this system could be useful for infor-
mation processing since it is a bistable system and can exhibit a binary logic behav-
ior. It should also be noted that, in the deprotonated rotaxane, it is possible to
displace the ring from the bipyridinium station by destroying the donor-acceptor
interaction through reduction of the bipyridinium station. Therefore, in this system,
mechanical movements can be induced by two different types of stimuli (acid base
and electron hole).
3.2.2
Molecular Shuttles Powered by Electrical Signals
A good example of an electrochemically driven molecular shuttle is rotaxane
8
reported in Fig.
6
. It consists of a benzylic amide macrocycle that surrounds an axle
featuring two hydrogen-bonding stations, namely, a succinamide (SA) and a naph-
thalimide (NI) unit, separated by a long alkyl chain (Brouwer et al.
2001
; Altieri
et al.
2003
). Initially, the ring resides onto the SA station (Fig.
6a
) because the NI unit
is a much poorer hydrogen-bonding recognition site. Electrochemical reduction of
the NI unit to the radical anion species can be carried out at −1.40 V versus the satu-
rated calomel electrode (Fig.
6b
). Since the naphthalimide anion is a much stronger
hydrogen-bonding station compared to the succinamide, thermal fl uctuations drive
the ring from the latter to the former station (Fig.
6c
). Successive electrochemical
oxidation (−0.90 V) of the naphthalimide anion back to the neutral state (Fig.
6d
) is