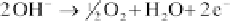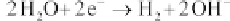Environmental Engineering Reference
In-Depth Information
Table 4.11
Energetic Data for Hydrogen in its Normal State
(
ρ
= 0.101 MPa,
T
= 273.15 K = 0°C)
Lower calorific value (LCV)
Upper calorific value (UCV)
Density
3.00 kWh/m
n
3
3.55 kWh/m
n
3
0.09 kg/m
3
(gaseous)
33.33 kWh/kg
39.41 kWh/kg
70.9 kg/m
3
(liquid, -252°C)
Note:
1 m
n
3
= 1 nominal cubic metre, equal to 0.09 kg of hydrogen gas
Battery backup systems can be connected to the grid with a photovoltaic
inverter. Photovoltaic generators can be combined with a wind generator or
diesel generator to create a hybrid system. This can reduce energy costs and
increase system availability; however, operating complex systems needs a
complex energy management system. If an AC load should be operated, an
inverter must be integrated into the system; inverters for photovoltaic systems
are described below.
Hydrogen storage and fuel cells
A promising option for future storage of large amounts of energy is hydrogen
(H
2
) storage (see energetic data in Table 4.11).
Electrolysis processes can produce hydrogen by using electricity as the
driving force. In alkaline electrolysis, water is split into hydrogen and oxygen
using two electrodes in a dilute alkaline electrolyte (see Figure 4.49).
The following equations describe the reactions:
Cathode:
(4.107)
Anode:
(4.108)
Alkaline electrolysis currently achieves efficiencies of 85 per cent. Besides
alkaline electrolysis, other methods for generating hydrogen from water exist
(e.g. membrane electrolysis or high-temperature vapour electrolysis). A smaller
Figure 4.49
Principle of Hydrogen Electrolysis with Alkaline Electrolyte