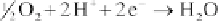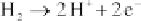Environmental Engineering Reference
In-Depth Information
Anode
Electrolyte
Cathode
not converted
H
2
not converted
O
2
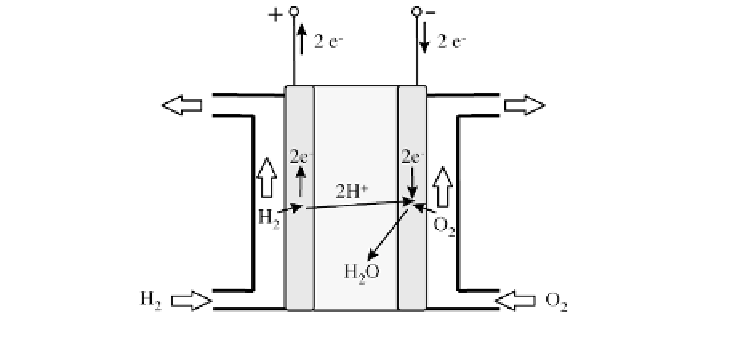
Figure 4.50
Principle of the Fuel Cell with Acid Electrolyte
amount of electric energy is sufficient to keep the process running at
temperatures above 700°C; prototypes of pressurized electrolysers have
already reached efficiencies of 90 per cent.
Gas and steam turbine power plants or fuel cells can generate electricity
from hydrogen. However,
fuel cells
are the most promising option to date. A
fuel cell with an acid electrolyte reverses the electrolysis by regenerating water
(H
2
O) from oxygen O
2
and hydrogen H
2
(see Figure 4.50); electrical energy is
a result of this reaction. The anode collects electrons that are released. The
hydrogen ions H
+
diffuse through the electrolyte to be collected at the cathode.
There they coalesce with the oxygen ions and electrons from outside to form
water molecules.
The equations of the reactions in the fuel cell are:
Anode:
(4.109)
Cathode:
(4.110)
To increase the voltage, several cells are connected in series into stacks (Figure
4.51).
The efficiency of an ideal fuel cell is 94.5 per cent. Today, efficiencies of up
to 80 per cent can be achieved. Fuel cells are divided into low-temperature,
mid-temperature and high-temperature cells. In addition to the electricity
generated, the heat from mid- and high-temperature fuel cells, such as the
phosphoric acid fuel cell (PAFC), the molten carbonate fuel cell (MCFC) and
the solid oxide fuel cell (SOFC), can be used. This increases the total efficiency.
The total efficiency
η
tot
of hydrogen storage is the sum of the electrical
efficiency
η
el
and the thermal efficiency
η
th
:
(4.111)