Chemistry Reference
In-Depth Information
2.8 Utilization of Voltammetry for
the Study of Organic
Electrosynthesis
2.8.1 Voltammetric Analysis for
Selective Electrosynthesis
Many organic electrode processes are in
principle multi-pathway, and therefore many
reaction products are usually obtained under
constant-current electrolysis. For example,
constant-current electrolysis for the reduction
of aromatic aldehydes or ketones under acidic
conditions gives two products, such as alcohols
and pinacols, simultaneously (
Eq. 2.7
) [10,11].
Consider linear sweep voltammetry (LSV) for
this reduction under mechanical stirring
(
Figure 2.7
). It is known that the first wave
corresponds to one-electron reduction of the
protonated substrate to form the corresponding
radical intermediate, while the second wave
corresponds to a second one-electron reduction
of the radical intermediate to form the alcohol,
as shown in
Eq. 2.7
. Therefore, when the
potential of the working electrode is maintained
at potential (known as the
constant-potential electrolysis), one-electron
reduction of the carbonyl compound will
proceed clearly to the radical intermediate and
consequently the pinacol product can be
obtained selectively. If, on the other hand, the
cathode is maintained at potential
, a
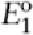
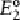
Search WWH ::

Custom Search