Chemistry Reference
In-Depth Information
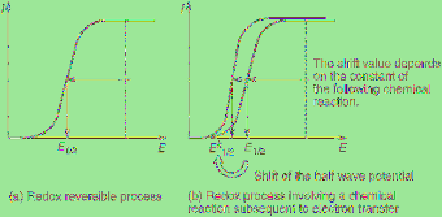
voltammetry is equal to half of the diffusion
limiting current (
Figure 2.6a
).
E
1/2
is equal to
the formal potential of the studied electrode
reaction when the reaction is a redox reversible
process and the diffusion coefficients of the
oxidized and reduced forms of a studied species
are equal to each other. However, if the reaction
involves a chemical reaction subsequent to
electron transfer,
E
1/2
is no longer equal to the
formal potential (
Figure 2.6b
).
Figure 2.6
Steady-state voltammograms of a
simple redox system (a) without and (b) with
following chemical reaction
The decomposition potential (
E
dec
) is a
potential where the Faradic current begins to be
observed on the voltammogram and is also
called the onset potential (
E
onset
). Although
E
dec
is often used as the thermodynamic index
for the oxidation/reduction of the studied
species, it is not exactly a thermodynamic
parameter.
Search WWH ::

Custom Search